Objective: To evaluate the long-term outcomes of children submitted to permanent cardiac pacing due to postoperative bradycardia and to identify risk factors for mortality.
Methods: From 1980 to 2004, 120 children were submitted to permanent pacemaker (PM) implantation. Interval between the defect correction and PM implantation was 1.2 ± 2.8 years, on average (median = 21 days). Atrioventricular blocks were present in 94.2% of patients. Transvenous leads (78.3%) and ventricular pacemaker systems (79.2%) were used in most of the cases. Risk factors were studied using the Cox proportional model. The Kaplan-Meier method and the Log-Rank test were used to analyze survival.
Results: After a mean of 5.7 ± 5.9 years (maximum = 22.5 years) of follow-up, 97 patients were alive and 11 were lost from the follow-up study. The main causes of death were terminal heart failure (10), infection not related to implantation (six), and sudden death (three). The 5-, 10-, and 15-year survival rates were 80.9 ± 4.1%, 75.4 ± 5.5% and 67.2 ± 7.4%, respectively. The persistence of hemodynamic problems (palliative procedures, the use of valve prostheses or the presence of residual defects) was identified as the only independent risk predictors for mortality, with significant alterations in the survival curves (p=0.0123).
Conclusion: The implant of permanent pacemakers in children provided good survival expectancy, mainly depending on the underlying disease and the type of the correction made. Palliative corrections, such as the presence of residual defects or valve prostheses were the only predictors of poor results in these children.
Objetivo: Avaliar a evolução tardia de crianças com marca-passo definitivo por bradicardia pós-operatória, e identificar fatores de risco para a mortalidade.
Método: De 1980 a 2004, 120 crianças receberam implante de marca-passo definitivo por bradicardia pós-operatória. O intervalo médio entre a correção do defeito e o implante foi de 1,2 ± 2,8 anos, com mediana de 21 dias. Bloqueio atrioventricular esteve presente em 94,2% dos pacientes. A via de acesso transvenosa (78,3%) e marca-passos (MP) definitivos ventriculares (79,2%) foram os mais utilizados. Empregou-se o método de Kaplan-Meier e o teste de Log-Rank para a análise de sobrevivência.
Resultados: Após 5,7 ± 5,9 anos de seguimento (máximo= 22,5 anos), 97 pacientes estavam vivos e 11 haviam sido perdidos para o seguimento. As principais causas de morte foram insuficiência cardíaca (10), infecção não relacionada ao marca-passo (seis) e morte súbita (três). A expectativa de sobrevida aos cinco, 10 e 15 anos de seguimento mostrou, respectivamente, índices de 80,9 ± 4,1%, 75,4 ± 5,5% e 67,2 ± 7,4%. A persistência de problemas hemodinâmicos após a correção (correções paliativas, uso de próteses valvares ou defeitos residuais) foi identificada como única variável preditora independente de risco para mortalidade, alterando significativamente as curvas de sobrevivência (p = 0,0123).
Conclusões: O implante de marca-passo em casos de bradicardia pós-operatória possibilitou boa expectativa de sobrevida. A realização de correções paliativas, assim como a presença de defeitos residuais ou de próteses valvares, foram os únicos fatores preditores de mau prognóstico para essas crianças.
INTRODUCTION
The use of definitive pacemakers (DPM) in children is rare, with surgical lesion of the conduction system being the main reasons for their implantation.
Permanent postoperative bradyarrhythmias have been associated to high morbidity due to arrhythmias with hemodynamic involvement. Postoperative atrioventricular blocks, in particular, are associated to high mortality even in patients submitted to corrections considered complete [1].
Permanent artificial heart pacing significantly reduces the death in these patients, giving an adequate long-term evolution. Mortality is generally related to the underlying disease and rarely to dysfunction of the pacing system.
The current study was developed aiming at analyzing the long-term evolution of patients with DPM due to postoperative bradyarrhythmias and to identify risk factors for death.
METHOD
In the period from November 1980 to April 2004, 120 patients with ages of up to 18 years old and with persistent postoperative bradyarrhythmias were submitted to the implantation of permanent PM in the Heart Institute (InCor) of the Medical School of the University of São Paulo (FMUSP).
At the time of the first PM implantations, ages varied from 43 days to 18 years with a mean of 5.9 ± 5.0 years and a median of 4.3 years. Sixty-five of the patients were female (54.2%) and 55 male (45.8%).
Bradycardia in isolation as the potential risk factor of sudden death justified implantation in 78 (65.0%) patients, signs and symptoms of heart failure in 22 (18.4%), syncopes in 10 (8.3%) and presyncopes in 10 (8.3%).
Persistent total atrioventricular blocks (TAVB) were the main electrographic finding (81.7%). Intermittent TAVBs (7.5%), 2nd-degree type-II atrioventricular blocks (5.0%) and different forms of sinusal node disease (5.8%) were present in the other patients.
The average time between correction of the defect that caused the lesion of the conduction system and PM implantation was 1.2 ± 2.8 years with a mean of 21 days. Only three patients were submitted to PM implantation concomitantly to the correction of the defect that caused bradycardia. PM implantation was performed up to 14 days after correction of the defect in 27.5% of the children, from 15 to 30 days in 30%, from one to six months in 18.3%, from six to 12 months in 3.3% and one year after the operation in 18.3%.
Correction of a defect of the submembranous interventricular septum in isolation was the most common procedure associated with injury of the electrical conduction system of the heart in 15 cases (12.4%) and together with other defects in 23 (19.0%) patients. Correction of an atrioventricular septum defect was performed in 18 (14.9%) and tetralogy of Fallot in 15 (12.4%) patients. Complex heart diseases were corrected in 25 (20.7%) children and patients with left atrial isomerism in 6.6%.
Correction of congenital heart defects was considered total and without residual defects or the need of valvar prostheses in 74 (61.7%) patients. Residual defects in hearts considered to be completely corrected were detected in 12 (19.2%) patients. Valvar prostheses were utilized after corrections considered complete in 23 (19.2%) patients, either in their anatomical position or in valved tubular grafts. Palliative corrections were performed in 11 (9.1%) cases.
One child submitted to palliative correction also received a valvar prosthesis.
Non-cardiac problems were identified in 11 children: Down Syndrome (9), Noonan Syndrome (1) and epilepsy (1).
Access routes utilized
In the first PM implantation procedures, 95 (79.2%) patients were submitted to the placement of a ventricular system, 18 (15.0%) received a atrioventricular PM, four (3.3%) multiple site systems and three (2.5%) atrial pacers.
Transvenous accesses were utilized in 94 (78.3%) children, by femoral (57.5%), subclavian (19.2%) or cephalic (1.6%) veins. Epimyocardial implantations were performed in 26 (21.7%) children and implants by transthoracic or subxyphoid approaches in 15.8% and 5.9%, respectively.
The pulse generator was implanted in the abdominal wall in 91 (75.8%) patients and in the thoracic wall in 29 (24.2%) either in the infraclavicular or submammary regions.
Studied risk factors
The influence of factors considered a risk for death were studied, including the ages of patients at the time of surgical correction and PM implantation, the gender, the persistence of hemodynamic problems after the correction (palliative corrections, the use of valvar prostheses or residual defects), the time interval between the surgical correction and PM implantation, the echocardiographic findings at the time of implantation and the access utilized for implantation.
Statistical analysis
The expected survival curve in relation to time was determined by the non-parametric Kaplan-Meier method. Evaluation of the evolution of patients submitted to heart transplantation or removal of the PM was ceased at the moment of these procedures. One patient submitted to the removal of the PM who received a new implantation five years after, was included in the survival curve twice. Exploratory analysis of the risk factors was achieved by the Cox proportional model. The differences between the frequency of events over time and the identified variables were compared using the Log-Rank test.
All the data were analyzed using the Statistical Package for Social Sciences software (SPSS), with a p-value < 0.05 considered significant.
RESULTS
Follow up of patients lasted, on average, 5.7 ± 6.0 years with a range of four days to 22.5 years giving a total of 679.1 patient-years of follow up. Eleven patients were lost during the follow-up period, the first case soon after PM implantation and the last 12.5 years after the procedure (mean 2.5 ± 1.0 years).
Reoperations to maintain the artificial heart pacing system were performed on 82 occasions, due to pulse generator exhaustion (51.2%), growth of the child (15.8%), problems related to electrodes (15.9%) and infection (4.9%). Changes to the pacing mode, due to heart insufficiency, were necessary in six (8.5%) patients: to atrioventricular mode in three, to atrio-biventricular in three and to atrio-bifocal of the left ventricle in one patient. Other less common reasons indicated reoperations in 3.7% of the patients.
After the initial DPM implantation, reoperations were performed on 20 patients: 13 corrections for residual defects, 11 valve replacements and two heart transplantations.
Recovery of the atrioventricular conduction occurred in only one child, when it was possible to stop to use the PM five months after implantation during an operation to close a residual interventricular communication and re-enlargement of the right ventricle outflow tract. Five years later however, this patient was again submitted to PM implantation due to intermittent total atrioventricular block.
Twenty-three patients (19.2%) died during the follow up. The earliest death occurred 14 days after PM implantation and the latest 13.5 years after the operation (mean 28 ± 3.9 years). The causes of death were terminal heart failure (10), infections not related to the PM implantation (6), sudden death (3) and thromboembolism (1). In three patients the cause of death was not determined.
The expected survival rates at 5, 10 and 15 years of follow up were 80.9 ± 4.1%, 75.4 ± 5.5% and 67.2 ± 7.4% respectively (Figure 1).
The exploratory analysis of risk factors, achieved using the Cox proportional model, identified persistence of hemodynamic problems after the correction (palliative corrections, use of valvar prostheses or residual defects) as the only independent predicting variable associated with risk of mortality. Figure 2 shows the survival curves of patients submitted to total correction versus incomplete correction or the presence of valvar prostheses. Analysis of these results gave significant differences (p-value = 0.0123). In patients without hemodynamic disorders, the relative risk of death was 0.36 (0.12 to 0.77; 95% confidence interval) indicating a reduction of risk for death of 64%, showing that the risk of mortality of patients submitted to palliative corrections, those with implanted valvar prostheses or residual defects was 2.75 greater than the patients submitted to total corrections.
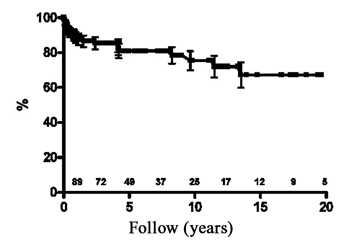
Fig. 1 - Analysis using the Kaplan-Meier method of the survival rate after pacemaker implantation
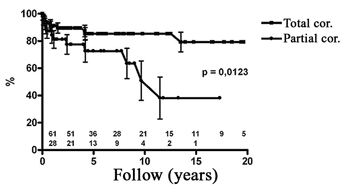
Fig. 2 - Evaluation of the survival rate of children submitted to total correction in comparison to those submitted to palliative corrections, those with residual defects or those using valve prostheses. (Partial correction = palliative, with residual defects or with valve prostheses)
The necessity of employing artificial heart stimulation in the pediatric population is not common with the main indication being surgical lesions of the conduction of the cardiac electrical stimulations during heart surgery. In cases where an irreversible atrioventricular block occurs, high mortality rates have been reported.
The epimyocardial access, by subxyphoid or transthoracic accesses particularly in patients of less than 15 kilograms, is the most common method of pediatric implantation reported in the literature. The transvenous access however has shown better long-term results, with lower rates of electrode fractures or loss of command by increasing the pacing threshold.
Although there are few data related to long-term follow ups of children with PMs, artificial cardiac pacing significantly reduces the mortality of these patients from 60 to 80% in non-treated patients to 25% in patients with definitive stimulation [2,3].
Data from six retrospective studies that involved 796 children showed postoperative bradycardia to be the cause of PM implantation in 40 to 84% of cases, with congenital atrioventricular blocks in 16 to 42% of cases. Independent of the cause of bradycardia, cardiac structural defects were identified in 44 to 87% of these patients. Epimyocardial implantations were performed in 69 to 100% of the procedures and the age range of the patients ranged from one day to 21 years with a mean of 4.1 years. In these studies, the post PM implantation mortality rate varied from 4.1 to 22.9% and the expected survival over five years ranged from 74 to 78% after a mean follow up of 2.4 to 11.9 years [4-9].
Complications of artificial cardiac pacing rarely threaten the lives of these children, with reports of death related to PM in 2 to 4% of cases [5,10,11].
The incidence of DPM implantation for postoperative bradycardia in the literature has been reported in between 2 and 3% of all corrections [12,13]. Weindling et al. [12] analysed the incidence of all 3rd degree atrioventricular blocks, including reversible blocks, in different heart diseases, finding an incidence of 17% after the correction of left ventricle outflow tract defects, 11% in corrections of transposition of the great vessels, 4% in the correction of interventricular septum defects and 3% in the correction of Tetralogy of Fallot, with the necessity of definitive implants in approximately 14% of the studied population.
The great number of patients analyzed in the present study, in which children with postoperative bradycardia were treated, together with the long follow-up period, gave very representative data, including the analysis of risk factors for death, an aspect that has never been reported in the literature for this type of problem.
The total expected survival rates observed of 80.9% at five years, 75.4% at ten and 67.2% at fifteen years of follow up were similar to those observed in children submitted to similar corrections but did not evolve with bradycardia or the necessity of artificial heart pacing [14-24].
The causes of death seen in the current study, with predominance of terminal heart failure and infectious processes not related to PM, corroborate published reports that suggest the little significance of complications of artificial heart pacing as a cause of death.
The identification of residual hemodynamic problems as the only independent risk factor for death, demonstrated in the current study, proves the supposition previously described by many authors that the mortality of children submitted to PM implantation is mainly related to underlying diseases [5,10,11].
These data also agree with those found in the literature on children submitted to corrections that do not evolve to persistent bradycardia: children submitted to total corrections present with a good long-term evolution in spite of the use of extra-anatomic non-valved tubes or persistence of ventriculo-arterial discordance. On the other hand, children submitted to palliative corrections, with residual defects after total corrections or those with valve replacements, either in the anatomic position or in valved tubular grafts, have presented with worse evolutions [14-25]. Spevak et al. [25] reported survival rates of 73% and 51% after one and five years of follow up respectively in children of up to five years old submitted to valve replacement.
CONCLUSIONS
The implantation of definitive artificial cardiac pacemakers in children with postoperative bradycardia allows a good survival rate depending mainly on the underlying disease and the type of correction performed. Palliative corrections as well as the existence of residual defects or the use of valvar prostheses were the only predictive factors of a bad prognosis for these children.
BIBLIOGRAPHIC REFERENCES
1. Bruckheimer E, Berul CI, Kopf GS, Hill SL, Warner KA, Kleinman CS et al. Late recovery of surgically-induced atrioventricular block in patients with congenital heart disease. J Interv Card Electrophysiol. 2002;6(2):191-5.
2. Lillehei CW, Sellers RD, Bonnabeau RC, Eliot RS. Chronic postsurgical complete heart block particular reference to prognosis, management, and a new P-wave pacemaker. J Thorac Cardiovasc Surg. 1963;46:436-56.
3. Fryda RJ, Kaplan S, Helmsworth JA. Postoperative complete heart block in children. Br Heart J. 1971;33(4):456-62.
4. Serwer GA, Mericle JM. Evaluation of pacemaker pulse generator and patient longevity in patients aged 1 day to 20 years. Am J Cardiol. 1987;59(8):824-7.
5. Kerstjens-Frederikse MW, Bink-Boelkens MT, de Jongste MJL, Homan van der Heide JN. Permanent cardiac pacing in children: morbidity and efficacy of follow-up. Int J Cardiol. 1991;33(6):207-14.
6. Rao V, Williams WG, Hamilton RH, Williams MG, Goldman BS, Gow RM. Trends in pediatric cardiac pacing. Can J Cardiol. 1995;11(11):993-9.
7. Sachweh JS, Vazquez-Jimenez JF, Schöndube FA, Daebritz SH, Dorge H, Muhler EG et al. Twenty years experience with pediatric pacing: epicardial and transvenous stimulation. Eur J Cardiothorac Surg. 2000;17(4):455-61.
8. Cohen MI, Bush DM, Vetter VL, Tanel RE, Wieand TS, Gaynor JW et al. Permanent epicardial pacing in pediatric patients: seventeen years of experience and 1200 outpatient visits. Circulation. 2001;103(21):2585-90.
9. Thomson JD, Blackburn ME, Van Doorn C, Nicholls A, Watterson KG. Pacing activity, patient and lead survival over 20 years of permanent epicardial pacing in children. Ann Thorac Surg. 2004;77(4):1366-70.
10. Esperer HD, Singer H, Riede FT, Blum U, Mahmoud FO, Weniger J. Permanent epicardial and transvenous single- and dual chamber pacing in children. Thorac Cardiovasc Surg. 1993;41(1):21-7.
11. Young D. Permanent pacemaker implantation in children: current status and future considerations. Pacing Clin Electrophysiol. 1981;4(1):61-7.
12. Weindling SN, Saul JP, Gamble WJ, Mayer JE, Wessel D, Walsh EP. Duration of complete atrioventricular block after congenital heart disease surgery. Am J Cardiol. 1998;82(4):525-7.
13. Bonatti V, Agnetti A, Squarcia U. Early and late postoperative complete heart block in pediatric patients submitted to open-heart surgery for congenital heart disease. Pediatr Med Chir. 1998;20(3):181-6.
14. Clapp SK, Perry BL, Farooki ZQ, Jackson WL, Karpawich PP, Hakimi M et al. Surgical and medical results of complete atrioventricular canal: a ten-year review. Am J Cardiol. 1987;59(5):454-8.
15. Driscoll DJ, Offord KP, Feldt RH, Schaff HV, Puga FJ, Danielson GK. Five- to fifteen-year follow-up after Fontan operation. Circulation. 1992;85(2):469-96.
16. Duster MC, Bink-Boelkens MT, Wampler D, Gillette PC, McNamara DG, Cooley DA. Long-term follow-up of dysrhythmias following the Mustard procedure. Am Heart J. 1985;109(6):1323-6.
17. Helbing WA, Hansen B, Ottenkamp J, Rohmer J, Chin JG, Brom AG et al. Long-term results of atrial correction for transposition of the great arteries: comparison of Mustard and Senning operations. J Thorac Cardiovasc Surg. 1994;1089(2):363-72.
18. Kyger ER 3rd, Frazier OH, Cooley DA, Gillette PC, Reul GJ Jr, Sandiford FM et al. Sinus venosus atrial septal defect: early and late results following closure in 109 patients. Ann Thorac Surg. 1978;25(1):44-50.
19. Manning PB, Mayer JE Jr, Sanders SP, Coleman EA, Jonas RA, Keane JF et al. Unique features and prognosis of primum ASD presenting in the first year of life. Circulation. 1994;90(5 Pt 2):II30-5.
20. Metcalfe J, Somerville J. Surgical repair of lesions associated with corrected transposition: late results. Br Heart J. 1983;50(5):476-82.
21. Ng R, Somerville J, Ross D. Ebstein's anomaly: late results of surgical correction. Eur J Cardiol. 1979;9(1):39-52.
22. Serraf A, Bruniaux J, Lacour-Gayet F, Sidi D, Kachaner J, Bouchart F et al. Anatomic correction of transposition of the great arteries with ventricular septal defect: experience with 118 cases. J Thorac Cardiovasc Surg. 1991;102(1):140-7.
23. Serraf A, Lacour-Gayet F, Bruniaux J, Ouaknine R, Losay J, Petit J et al. Surgical management of isolated multiple ventricular septal defects: logical approach in 130 cases. J Thorac Cardiovasc Surg 1992;103(3):437-43.
24. Turley K, Hanley FL, Verrier ED, Merrick SH, Ebert PA. The Mustard procedure in infants (less than 100 days of age): ten-year follow-up. J Thorac Cardiovasc Surg. 1988; 96(6):849-53.
25. Spevak PJ, Freed MD, Castaneda AR, Norwood WI, Pollack P. Valve replacement in children less than 5 years of age. J Am Coll Cardiol. 1986;8(4):901-8.


 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license