INTRODUCTION
Several factors may influence mechanical respiration and gas exchange in heart surgeries increasing the risk of postoperative pulmonary complications (PPCs). Mechanical respiration is evaluated by measuring the dynamical compliance (DynC) and static compliance (StaC) and airway resistance (AWR) and gas exchange using the gas exchange index. Pulmonary complications are also related to risk factors in the preoperative period. The main risk factors studied in the preoperative period are advanced age, prior pulmonary diseases, smoking, bad nutritional state, altered pulmonary function and associated comorbidities; factors which lead to alterations in the integrity of respiratory system and can compromise mechanical respiration and gas exchange [1].
Preoperative risk factors, when associated to surgeries, can compromise mechanical respiration and gas exchange index in the immediate postoperative period, measurement of which may be useful to detect possible PPCs. PPCs are responsible for 40% of deaths in over 70-year-old patients because of respiratory function changes which include pulmonary compliance loss, increases in pulmonary resistance and reduction of gas exchange resulting from aging [2]. While it is possible to wait for improvements of acute respiratory diseases in elective surgeries, this is not possible with chronic diseases. Chronic respiratory diseases increase the numbers of pulmonary complications and when associated to respiratory symptomatology, elevate the risk of PPCs [3]. Smoking increases the risk of PPCs depending on the quantity of cigarettes smoked. Cigarettes have noxious effects that are responsible for PPCs and so 8 weeks of abstinence is fundamental to reduce these effects [4,5]. Malnutrition and obesity are also considered risk factors; the first leads to a reduction in the response to hypoxia of the muscle mass and to pulmonary defense due to proteic-caloric deficiency [3,6]; the second makes atelectasis more common and is responsible for a reduction in pulmonary compliance. Thoracic resistance, due to an increase of adipose tissue in this region, may lead to an increase in airway resistance [3,6]. Comorbidities such as arterial hypertension and diabetes mellitus are related to a higher risk of PPC and it is essential that these diseases are controlled in patients submitted to surgery.
The occurrence of PPCs may also be linked to factors related to anesthesia, such as the type and the time of anesthesia and the agent used [1,7], as well as to surgical factors related to the site of the incision, surgical type and intra-operative mechanical ventilation [1,7,8]. In heart surgeries there may be a necessity to employ cardiopulmonary bypass (CPB); responsible for the development of systemic and pulmonary inflammatory responses with the latter leading to mechanical respiratory dysfunction [9]. Patients who undergo heart surgery need mechanical ventilation in the postoperative period when the mechanical properties and the gas exchange index may be evaluated and utilized as criteria for extubation. It is known that patients who present preoperative risk factors have more complications in the postoperative period and that the complications in the postoperative period can be consequent to alterations in mechanical respiration and gas exchange.
The aim of this study was to compare values obtained for mechanical respiration variables and the gas exchange index in the postoperative period of on-pump heart surgery with the parameters normality reported in the literature. Secondly, to compare groups that were considered to have higher and lower risks in the preoperative period in respect to mechanical respiratory variables and gas exchange index.
METHOD
This research was approved by Ethics Research Committee of UNESP in Botucatu (number 137/2003CEP). Consecutive patients, candidates for coronary artery bypass grafting surgery (CABG) admitted to the Hospital de Base in Bauru, São Paulo State by one of the heart surgery teams, were studied in the period between August 2003 and June 2004. The patients were studied in the preoperative and in the immediate postoperative periods. The evaluation of the preoperative period was performed using a specific questionnaire in an interview always carried out by the same interviewer. The items on the questionnaire were related to risk factors for PPCs. The patient's history included prior pulmonary disease, respiratory symptomatology and number of cigarettes smoked. Prior respiratory diseases, the presence of acute or chronic pulmonary diseases, were also considered. Respiratory disease symptoms investigated were coughs, expectoration, dyspnea and bronchospasm.
The presence of at least one symptom was sufficient to classify the patient as symptomatic. Smoking was classified by the quantity of cigarettes smoked and the patients were divided into three groups; smokers, ex-smokers and non-smokers [1]. The nutritional state was evaluated using the Body Mass Index (BMI). The weight (Kg) was checked using a digital balance (FILIZOLA®) and the height was obtained using a anthropometer (cm). We considered the patients to be eutrophic when their BMIs were between 21 and 25 kg/cm2 and dystrophic with BMIs of less than 21 or more than 25 kg/cm2 [1]. The presence of comorbidities was also checked. Exclusion criteria included surgical procedures without the use of CPB, surgical access other than median sternotomy, total anesthesia time of less than 210 minutes, position during the procedure different to decubitus dorsal, spontaneous respiration in the immediate postoperative period and emergency surgeries. These data were included on a data collection questionnaire from the hospital records of patients and, when necessary, the surgical team was contacted.
Patients that fulfilled the inclusion criteria for the study received mechanical respiratory ventilation (Inter 5® Model I, Intermed maker) with a flow volume (FV) of 8 mL/kg of the ideal weight (previously calculated using the ideal BMI of men of 23 and women of 22 kg/m2), with an inspiratory flow of 0.83 L/s, respiration frequency of 16 cycles/min (maintaining the inspiration/expiration ratio greater than 1:2), PEEP of zero and after at 5 cmH2O and inspired oxygen fraction of 100%. Patients were maintained sedated using midazolam and fentanyl Citrate. After at least 15 minutes of ventilation, the peak pressure (PP) and plateau pressure (plP) were measured. The plP was obtained after a post-inspiratory pause by occlusion of the expiratory branch and the PP was considered the highest point registered by a digital manometer. Also after 15 minutes, blood from the right radial artery was collected for blood gas measurement (Hemogasometro Rapid Lab 860®).
The DynC (FV/PP-5), StaC (FV/plP-5), AWR (PP-plP/0.83) and gas exchange index were calculated. The patients were divided into groups based on qualitative preoperative variables, according to the gender, age (over 70-year-olds and under 70-year-olds), BMI (dystrophic and eutrophic), prior pulmonary disease, respiratory disease symptoms and comorbidities (absence and presence) and smoking classification (non-smokers, ex-smokers and smokers). These groups were then compared in relation to the postoperative variables (DynC, StaC, AWR, PaO2/FiO2). The qualitative variables were correlated using the Mann-Whitney non-parametric test, the variables DynC, StaC and PaO2/FiO2 were presented as means and standard deviations and the AWR presented as medians and interquartile intervals. Finally, the postoperative variables were compared with parameters considered normal by the proportion Test. The tests were considered significant when p-value < 0.05.
RESULTS
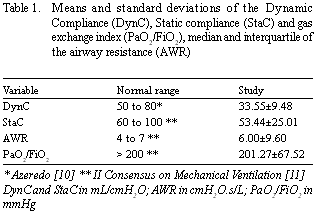
Seventy patients with ages varying from 26 to 77 years were evaluated, with 60 (85.7%) under 70-year-olds and a majority of male patients (61%). The weight mean, height and BMI were 68.55 ± 14.46 kg, l.64 ± 0.09 m and 25.31 ± 4.63 Kg/m2, respectively, with 43% eutrophic and 57% dystrophic. Eighteen patients (26%) were reported as having prior pulmonary diseases, 45 (64%) had respiration disease symptoms and 53 (76%) were suffering from comorbidities. Thirty-three patients (47.1%) were non-smokers, 20 (28.6%) ex-smokers and 17 (24.3%) smokers. The patients were taken to the intensive care unit on mechanical ventilation with a mean FV of 491.93 ± 62.24 mL, giving a mean PP and plP of 20.47 ± 3.45 cmH2O and 15.13 ± 2.42 cmH2O, respectively. The calculations of DynC, StaC and AWR were 33.55 ± 9.48 mL/cm H2O, 53.44 ± 25.01 mL/cm H2O and 6.41 ± 2.86 cmH2O/L/s, respectively. The mean gas exchange index was 201.27 ± 67.52 mmHg. Comparing the variables obtained in the immediate postoperative period with the values considered normal [10,11], it was seen that the mean DynC and StaC were lower than normal, while that the median of the AWR was within the normality range. In respect to the gas exchange index, although the mean was within the normal range, 47.14% of the patients presented with lower values (Table 1).
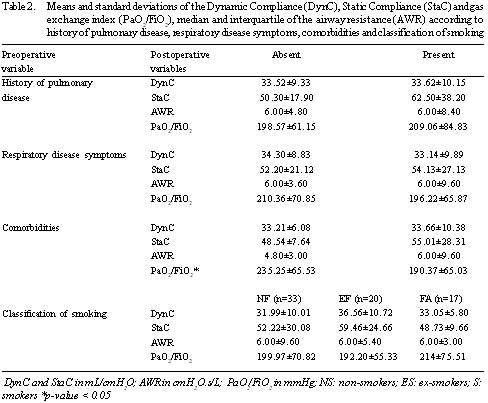
The results of the postoperative variables correlated to the risk factors are presented in Table 2. There was non-significant difference between the groups of patients who had had prior pulmonary diseases or not, those with and without respiratory disease symptoms and between non-smokers, ex-smokers and smokers. Only the groups with and without comorbidities presented a difference in the PaO2/FiO2 index with the patients who reported comorbidities having a lower ratio than those who did not have comorbidities.
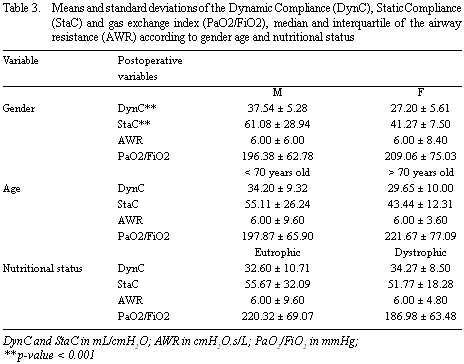
The DynC and StaC were significantly higher in men but there were no significant differences in the other postoperative variables in respect to gender. There was also no significant difference between over and under 70-year-old patients or between eutrophic and dystrophic patients (Table 3).
DISCUSSION
The compliance was lower in most patients. These changes may be specifically attributed to the intraoperative proceedings, including mechanical ventilation that uses low pulmonary volumes and low levels of PEEP, thereby helping to diminish the StaC. Sternotomy principally modifies the compliance of the rib cage, which reduces its mobility by more than 80% for up to 7 days after the surgery. This leads to atelectasis, reducing the DynC [8]. CPB may be responsible for changes in the StaC. Pulmonary compliance decreases after CPB due to the accumulation of liquids in the pulmonary interstice and to the inflammatory response [9,12]. Auler Junior et al. [13] and Nozawa et al. [14] also reported reductions in the StaC in the immediate postoperative period. Polese et al. [15] studying airway resistance and elastance, that is, the inverse of compliance, also found a reduction in the compliance and an increase in airway resistance after heart surgery with CPB. It is known that patients submitted the CABG surgery present with a reduction in the gas exchange index when compared to preoperative values [16]. Patients after heart surgery are benefited from alveolar recruitment with the utilization of PEEP improving the gas exchange index [13]. Almost half of patients needed therapy to reverse hypoxemia, which included alveolar recruitment, increases in PEEP levels or oxygen therapy.
Patients with pulmonary diseases presented with reduced compliance in relation to normal, but did not significantly differ from patients without pulmonary diseases. This leads us to believe that intraoperative factors were the main cause of alterations in mechanical respiration in the immediate postoperative period. Mechanical ventilation of patients with obstructions in the intraoperative period with low flow volumes and low PEEP levels, avoids hyper-insufflation and auto-PEEP processes; but, favors the formation of atelectasis and ventilation-perfusion disorders [17]. This fact would explain the reduction of the compliance in both groups. CPB may cause respiratory dysfunctions frequently with atelectasis [16], a situation aggravated by the manipulation of the chest and by the decubitus. These lead to a reduction of compliance that can persist up to six days [18]. Barbosa and Carmona [16] evaluated the StaC in patients submitted the heart surgery and also found a reduction in the immediate postoperative period. The deleterious effects of CPB on the mechanical respiration of patients with a history of pulmonary disease are summed to the increase in the expected StaC of these patients. Auler Junior et al. [13] also using a PEEP of 5 cmH2O obtained a gas exchange index similar to ours in patients submitted to CABG. The gas exchange index decreased by 59% in relation to the preoperative period. It improved by the second postoperative day, but did not return to the initial values [16,19].
Of the 45 patients who presented with symptoms, only one third reported a history of respiration disease, showing the importance of checking the symptoms of all patients. But there were no significant differences between groups with and without symptoms in respect to the mechanical properties or the gas exchange index, in spite of the mean gas exchange index of the symptomatic group being considered hypoxic. This, in patients after CABG, reduces in relation to the preoperative period probably due to induction of anesthesia and the effects of CPB on the pulmonary interstice [16]. Auler Junior et al. [13] verified that the PEEP increases the compliance, reduces the resistance and increases the gas exchange index in hypoxemic patients after heart surgery. In our study, patients with respiratory disease symptoms present gas exchange indexes of less than 200 cmH2O even with PEEP; thus, these are patients who merit an ideal PEEP with the aim of improving alveolar recruitment in the postoperative period to revert the hypoxemia.
There was also no difference in the mechanical respiratory variables for the groups with and without comorbidities; however, in the group with comorbidities, the gas exchange index was significantly lower. The gas exchange index is one of the parameters used as extubation criterion [16]. Diabetic patients have the mechanical ventilation time increased [20]. This may be one of the reasons that the gas exchange index was reduced in our patients as diabetes was one of the most frequent comorbidities evidenced. In patients who undergo heart surgery, gender is considered to be a risk factor as female patients are operated on at an older age, thereby presenting with comorbidities related to age [21,22].
The significant differences of the compliance between genders may have occurred, as the lungs of men are larger than those of women. When we utilized a specific compliance, which is a manner to compare compliance of different sized lungs, there was no significant difference between genders. With the gas exchange index, no significant difference was found between genders too. Some authors have found greater mechanical ventilation times for women [20,23]. Both genders presented with reduced mechanical respiratory variables, but these did not seem to alter the decision of extubation and consequently the mechanical ventilation time.
Significant differences were not evidenced between the groups of over and under 70-year-olds. We believe that this happens mainly because the group of over 70-year-olds was much smaller. Other important characteristics, which were not investigated in this study, are the comorbidities related to the age of patients. Elderly patients presented with lower mean DynC, in spite of the difference being non-significant. The drop in the DynC with age is easily explained by characteristics of aging of the chest which leads to rigidity and thus reduces the DynC. Nevertheless, in the two groups, the DynC was lower than normal probably due to intraoperative alterations, including the incision, the CPB and the intraoperative position. The measurement of StaC in elderly patients is frequently normal or increased because of pulmonary aging and senile emphysema. Significant differences in the StaC with age were not identified and were lower than normal values in both groups.
It seems that the factors mostly responsible for changes in the StaC are those seen in the intraoperative period. It is known that what determine the greatest postoperative pulmonary complications in the elderly are alveolar instability and the greater susceptibility to form atelectasis [24]. Elderly patients also present with a reduction in respiratory muscle force which may increase the ventilation time and after extubation, make re-expansion of areas of atelectasis difficult, contributing to the occurrence of complications [2]. Finally, there was no significant difference in the gas exchange index with age, even though the younger group presented with mean values of less than 200 mmHg. The nutritional state is a parameter that has been evaluated in patients submitted to CABG [16], as malnutrition leads to a reduction in the response to hypoxia, diminishing the muscle mass and decreasing the pulmonary defense by proteic-caloric deficiency [3,6].
An increase in weight leads to alterations in mechanical respiration leading to atelectasis [6,25,26]. Morbidly obese patients, compared to normal patients, when submitted to abdominal surgery, present with a StaC reduced by a half and AWR increased by almost three times [25]. In our study, although the StaC was lower in dystrophic patients, the difference was not significant; but it is important to remember that the dystrophic group was composed of overweight and underweight patients and not of morbidly obese patients. Our results were similar to those of Barbosa & Carmona [16] in overweight patients submitted to CABG. There were no statistically significant differences between the eutrophic and dystrophic groups in relation to the gas exchange index; however in dystrophic patients the mean was less than 200 mmHg, a finding attributed to the possible existence of atelectasis and a reduction in the response to hypoxemia. It is believed that obese patients present with greater areas of atelectasis and that it is not possible improve this by maintaining the PEEP close to the physiological level (from 3 to 5 cmH2O). Eutrophic patients seem to be benefited from PEEP, even with low values, improving the exchange area and consequently the gas exchange index.
Smoking is a factor of great importance in the preoperative evaluation as it increases the pulmonary complications [4,5]. In our study there were no significant differences between the groups, all presented with mechanical respiration variables and gas exchange indexes lower than normal. We believe that there was no difference because smokers ceased the habit for an average of seven weeks before the surgery, a period sufficient to reduce surgical risks [5]. In spite of not finding significant differences among the groups, it is known that smokers present a higher risk of pulmonary complications in the postoperative period, as these patients do not present with integrity of the respiratory system sufficient to maintain alveolar ventilation after extubation [18]. In order to improve the evolution of these patients, several procedures may be used including physiotherapy in the preoperative and postoperative periods, medicinal therapy and the maintenance of postoperative mechanical ventilation [6,21].
CONCLUSION
Independently of risk factors, compliance is decreased in almost all operated patients and airway resistance increased in a third. The gas exchange index is diminished in half of these patients. The study also showed that, in the immediate postoperative period of patients submitted to CABG surgery, the risk factors did not influence the mechanical ventilatory variables or the gas exchange index. This latter was altered only in the patients who presented with comorbidities.
ACKNOWLEDGEMENTS
The authors wish to thank Prof. Carlos Roberto Padovani PhD for the statistical analysis of the results and the Directors of Hospital de Base de Bauru for giving permission to study their patients.
BIBLIOGRAPHIC REFERENCES
1. Pereira EDB, Fernandes ALG, Anção MDS, Peres CDAP, Atallah APAN, Faresin SM. Prospective assessment of the risk of postoperative pulmonary complications in patients submitted to upper abdominal surgery. São Paulo Med J. 1999;117(4):151-60.
2. Fernandes CR, Ruiz Neto PP. O sistema respiratório e o idoso: implicações anestésicas. Rev Bras Anestesiol. 2002;52(4):461-70.
3. Saad IAB, Zambom L. Variáveis clínicas de risco pré-operatório. Rev Assoc Med Brás. 2001;47(2):117-24.
4. Bluman LG, Mosca L, Newman N, Simon DG. Preoperative smoking habits and postoperative pulmonary complications. Chest. 1998;113(4):883-9.
5. Nakagawa M, Tanaka H, Tsukuma H, Kishi Y. Relationship between the duration of the preoperative smoke-free period and the incidence of postoperative pulmonary complications after pulmonary surgery. Chest. 2001;120(3):705-10.
6. Doyle RL. Assessing and modifying the risk of postoperative pulmonary complications. Chest. 1999;115(5 suppl.):77S-81S.
7. Chumillas S, Ponce JL, Delgado F, Viciano V, Mateu M. Prevention of postoperative pulmonary complications through respiratory rehabilitation: a controlled clinical study. Arch Phys Med Rehabil. 1998;79(1):5-9.
8. Ragnarsdóttir M, Kristjánsdóttir Á, Ingvarsdóttir I, Hannesson P, Torfason B, Cahalin L. Short-term changes in pulmonary function and respiratory movements after cardiac surgery via median sternotomy. Scand Cardiovasc J. 2004;38(1):46-52.
9. Massoudy P, Zahler S, Becker BF, Braun SL, Barankay A, Meisner H. Evidence for inflammatory responses of the lungs during coronary artery bypass grafting with cardiopulmonary bypass. Chest. 2001;119 (1):31-6.
10. Azeredo, CAC. Modificações da constante de tempo durante a ventilação mecânica: um desafio permanente. In: Ventilação mecânica invasiva e não invasiva. Rio de Janeiro:Revinter;1994. p.1-48.
11. Consenso Brasileiro de Ventilação Mecânica (II). J Pneumologia. 2000;26(2):16-20.
12. Huang H, Yao T, Wang W, Zhu D, Zhang W, Chen H et al. Continuous ultrafiltration attenuates the pulmonary injury that follows open heart surgery with cardiopulmonary bypass. Ann Thorac Surg. 2003;76(1):136-40.
13. Auler Júnior JO, Carmona MJ, Barbas CV, Saldiva PH, Malbouisson LM. The effects of positive end-expiratory pressure on respiratory system mechanics and hemodynamics in postoperative cardiac surgery patients. Braz J Med Biol Res. 2000;33(1):31-42.
14. Nozawa E, Kobayashi E, Matsumoto ME, Feltrim MI, Carmona MJ, Auler Júnior JO. Avaliação dos fatores que influenciam no desmame de pacientes em ventilação mecânica prolongada após cirurgia cardíaca. Arq Bras Cardiol. 2003;80(3):301-10.
15. Polese G, Lubli P, Mazzucco A, Luzzani A, Rossi A. Effects of open heart surgery on respiratory mechanics. Intensive Care Med. 1999;25(10):1092-9.
16. Barbosa RAG, Carmona MJC. Avaliação da função pulmonar em pacientes submetidos à cirurgia cardíaca com circulação extracorpórea. Rev Bras Anestesiol 2002;52(6):689-99.
17. Vieira JE, Silva BAR, Garcia Júnior D. Padrões de ventilação em anestesia: estudo retrospectivo. Rev Bras Anestesiol. 2002;52(6):756-63.
18. Ferreira Júnior FH, Boas AGV, Gonnelli CA, Puig LB, Stolf N. Assistência ventilatória mecânica em pós-operatório de cirurgia cardíaca. An Paul Med Cir. 2003;130(1):22-30.
19. Taggart DP, El-Fiky M, Carter R, Bowman A, Wheatley DJ. Respiratory dysfunction after uncomplicated cardiopulmonary bypass. Ann Thorac Surg. 1993;56(5):1123-8.
20. Branca P, McGaw P, Light R. Factors associated with prolonged mechanical ventilation following coronary artery bypass surgery. Chest. 2001;119(2):537-46.
21. Schüller D, Morrow LE. Pulmonary complications after coronary revascularization. Curr Opin Cardiol. 2000;15(5):309-15.
22. Woods SE, Noble G, Smith JM, Hasselfeld K. The influence of gender in patients undergoing coronary artery bypass graft surgery: an eight-year prospective hospitalized cohort study. J Am Coll Surg. 2003;196(3):428-34.
23. Price JA, Rizk NW. Postoperative ventilatory management. Chest. 1999;115(5 suppl):130S-7.
24. Medeiros AC, Rocha KBF, Dantas Filho AM, Aires Neto T, Pinto JR Fel, Medeiros BC. Repercussões do tempo operatório em pulmões de ratos idosos. Acta Cir Bras 2003;18(Suppl. 1):40-4.
25. Pelosi P, Croci M, Ravagman I, Vicardi P, Gattinoni L. Total respiratory system, lung and chest wall mechanics in sedated-paralysed postoperative morbidly obese patients. Chest. 1996; 109(1):144-51.
26. Platell C, Hall JC. Atelectasis after abdominal surgery. J Am Coll Surg. 1997;185(6):584-92.
 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license