INTRODUCTION
In spite of the great development in surgical techniques of off-pump coronary artery bypass grafting, myocardial protection has not lost its importance due to high number of cases in which it is of extreme necessity. Hence, research on myocardial protection continues to be published worldwide. The advantages of sanguineous cardioplegia are well established [1,2] and have been responsible for the reduction of surgical mortality rates with high-risk patients [3]. Several studies have shown better clinical results using continuous sanguineous cardioplegia [4-7] and retrograde perfusion through the coronary sinus [8,9]. The method of retrograde cardioplegia is considered better for patients with significant injury of the left coronary artery trunk and in patients submitted to redo coronary artery bypass grafting (CABG) [10,11]. Clinical works on myocardial protection rarely identified significant differences in the results [12,13], however works using metabolic analysis are important as it is possible to verify small differences in the results.
The objective of this study is to determine alterations suffered by the myocardium, utilizing continuous retrograde hypothermic sanguineous cardioplegia in addition to normothermic anterograde cardioplegia induction. The aim of this technique is to better preserve myocardium ATP reserves during the asystole induction phase or even to re-establish these reserves if there is a preexistent depletion [14,15].
METHOD
Patients
Fifteen patients electively referred for CABG and who accepted to participate in this study were evaluated. The study was approved by the Medical Ethics Committee of the hospital. Inclusion criteria were obstructive coronary disease involving two or three arteries and an ejection fraction of less than 40%. The exclusion criteria were unstable angina, insulin-dependent diabetes and associated surgeries (endarterectomy, left ventricular aneurysmectomy, valve replacement etc.). All patients were operated on by the same surgeon (CGS).
Operative technique
The anesthesia technique employed was the same for all patients. After median sternotomy and dissection of the left internal thoracic artery, the patients were heparinized, the pericardium was opened and the aorta and right atrium were cannulated using a 22F arterial cannula (DLP®) and a two-stage venous cannula (DLP®), respectively. The heart-lung machine was manufactured by Stöckert® and the membrane oxygenator by Dideco ®. Hemodilution (perfusate of 2000 mL of Ringer lactate solution) and systemic hypothermia, maintaining the rectal temperature between 28-30 ºC, were used.
Myocardial protection
A retrograde cardioplegia catheter (DLP®) was introduced in the coronary sinus through a hole in the right atrial wall. The pressure of retrograde cardioplegia was continuously monitored and maintained below 50 mmHg. A small needle-like thermometer was introduced in the apex of the left ventricle for continuous monitoring of the myocardial temperature. A special thermal isolator was used between the heart and the diaphragm to protect against possible thermal injury to the phrenic nerve and ice slush was placed on the heart. Oxygenated blood coming from the oxygenator through a Y-shape line was mixed with potassium chlorate solution at a proportion of 4:1 giving a blood potassium concentration of 20 mmol/L and a hematocrit concentration of 22%. The dose of cardioplegic induction was performed with an infusion of 750 mL of anterograde normothermic sanguineous cardioplegia (37 ºC) through the anterograde cardioplegia cannula in the aortic root, followed by an infusion of 500 mL of cold sanguineous cardioplegia at a temperature of 4-6 ºC infused in the coronary sinus, at a velocity of 200-300 mL/min, under a pressure of 50 mmHg. After induction, the concentration of potassium of the cardioplegia was altered to 10 mmol/L (proportion 8:1) and maintained at a temperature of 4-6 ºC and the solution was continuously infused in the coronary sinus at a velocity of 50-75 mL/min under a pressure of 50 mmHg.
When necessary, saline irrigation was used to simplify the distal anastomoses improving the vision of the artery edges, as continuous bleeding through the coronary arteries sometimes hampers visibility. Reheating was initiated during the last distal anastomosis and the proximal anastomoses of the aorta were performed with the help of aortic clamping.
Measurements
Samples of arterial blood (a) and blood from the coronary sinus (cs) were collected for analysis of oxygen and lactate concentration. The samples were simultaneously collected: before establishing the CPB, at the end of normothermic anterograde induction, on opening the aorta and after 10, 30 and 60 minutes of reperfusion (due to technical problems, analysis of the samples collected after cardioplegic induction was performed for only eight patients). With a special pistol (Biopsy-Cut ®), four myocardial biopsies were obtained from the apex of the left ventricle: (1) after establishing the CPB (but before aortic clamping), (2) immediately after the end of cardioplegic induction, (3) before aortic declamping and (4) after 30 minutes of reperfusion. The biopsies were immediately frozen in liquid nitrogen and stored at -80 ºC until analysis. On the day of analysis, the biopsies were cleaned of fat, blood and connective tissue by macroscopic dissection with the temperature (22º C) and humidity (30%) constant and extracted in 0.5 M of perchloride acid. The acid was removed and neutralized using 2 M of KHCO3 and analyzed by enzymatic fluorometry methods to determine the concentrations of adenosine triphosphate (ATP), adenosine diphosphate (ADP), adenosine monophosphate (AMP), total adenosine nucleotides (TAN) defined as (ATP+ADP+AMP) and lactate. The ATP/ADP ratio was also calculated.
The CK-MB isoenzyme was analyzed in venous blood samples taken before CPB and 1, 3, 6, 9, 12 and 24 hours after aortic declamping. A method of mass spectrometry (IMx STAT CK-MB, Abbot laboratories, Abbot Park, IL 60064, USA) that is highly sensitive was utilized to determine the MB fraction. The invasive pressures of the radial artery and right and left atria were used for postoperative hemodynamics monitoring.
Statistical analysis
The student t-test was used for statistical analysis. Significant differences were defined as the probability of p-value < 0.05 for each test. The values are presented as means and standard deviations.
RESULTS
Clinical results
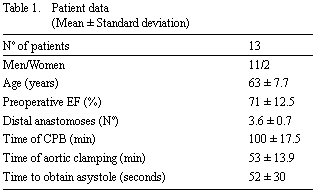
Two patients were eliminated from the study due to displacement of the catheter from the coronary sinus. The ages of the patients, genders, ejection fractions (EF), number of peripheral anastomoses, time of aortic clamping and of CPB and time to obtain asystole are illustrated in Table 1. Thirty per cent of the patients (four patients) had histories of prior AMI. No deaths occurred in the group. No patient needed inotropic support. There was no perioperative or postoperative AMI (elevation of enzymatic levels followed by the appearance of a Q-wave in at least two derivations). One patient was reoperated due to instability of the sternum but afterwards evolved well. Six (46%) patients presented with atrial fibrillation during the hospital stay.
Metabolic results
Lactate metabolism
Sequential measurements of the sanguineous myocardial lactate (arterial and of the coronary sinus) collected before CPB, at the end of normothermic cardioplegic induction, on opening the aorta and 10, 30 and 60 minutes after the initiation of reperfusion are shown in Figure 1 (negative values indicate lactate production). Before CPB the difference (a-cs) of lactate was +0.04 mmol/L. At the end of normothermic cardioplegic induction this value increased to +0.09 mmol/L, a statistically non-significant increase. At the start of reperfusion, there was a change in lactate release giving a difference of -0.22 mmol/L which progressively increased to -0.79 mmol/L at 10 minutes of reperfusion and at 30 minutes of reperfusion this difference was -0.25 mmol/L (all differences were statistically significant). Only after 60 minutes of reperfusion, the lactate arterial-venous difference had returned to the initial levels (+ 0.03 mmol/L).
Oxygen metabolism
Calculations of the arterial-coronary sinus (a-cs) sequential blood oxygen differences performed before CPB and on finishing normothermic cardioplegic induction, on opening the aorta and after 10, 30 and 60 minutes of reperfusion are displayed in Figure 2. The a-cs oxygen saturation difference before CPB was 108 mL/L. At the end of normothermic cardioplegic induction this value fell to only 10 mL/L. When the aorta was declamped, the difference was only 18 mL/L. This low oxygen difference increased to 84 mL/L after 60 minutes of reperfusion; still lower than the initial value (all the measurements compared to the control are statistically significant).
Myocardial biopsies
The first myocardial biopsy performed before aortic clamping, shows that the ATP in the heart muscle was 22.6 mg/g of dry muscle. The two biopsies performed during aortic clamping, immediately after cardioplegic induction and before declamping, showed levels of 20.2 and 21.4 mg/g, respectively. After thirty minutes of reperfusion there was a reduction to 18.1 mg/g. The ATP/ADP ratio, which is considered a cellular metabolic function marker, was also analysed. The results are illustrated in Table 2.
The control lactate level in the myocardium before CPB was 13.20 mg/g of dry muscle. The two biopsies attained during aortic clamping gave values of 16.14 mg/g immediately after cardioplegic induction and 23.90 mg/g before aortic declamping (p-value < 0.01). After 30 minutes of reperfusion, the level of lactate in the muscle had diminished to 19.67 mg/g, which is still statistically significant when compared with the control biopsy (p-value < 0.01). The results are shown in Figure 3.
CK-MB enzyme serum levels
The peak CK-MB enzyme serum level occurred six hours after aortic declamping. The results are illustrated in Table 3 and Figure 4.
COMMENTS
From the clinical point of view, the results obtained in the study were good. There was a reduction in the time to obtain asystole with the start of normothermic induction in comparison to a previous work [16]. This can considerably reduce the ATP loss during the period to obtain the end of electromechanical activity. The metabolic results were very similar to a previous work [16] using the same cardioplegic method, but without anterograde normothermic cardioplegic induction. A high incidence of postoperative atrial fibrillation (46%) was observed.
Lactate metabolism
Similar to our earlier study [16], the lactate arterial-coronary sinus difference before CPB showed a normal concentration of lactate. The myocardium, in normal aerobic conditions, uses lactate in the production of energy (ATP) and releases it during anaerobic metabolism. The a-cs difference of lactate at the end of normothermic cardioplegic induction was positive, suggesting the utilization of the lactate as an energy source. But the O2 arterial-venous difference in this period was insignificant, creating doubts about how the myocardium can utilize lactate as an energy source in practically anaerobic conditions. After aortic declamping there was a release of lactate. This can result from both an abnormal production at that time or an earlier accumulation during aortic clamping or even both hypotheses, indicating some degree of anaerobic metabolism at the start of reperfusion. However, the continuous retrograde hypothermic sanguineous cardioplegia seems not to have released enough oxygen to cells, probably due to the low temperature [17] or due to regions of the heart that were not satisfactorily perfused by the retrograde cardioplegia. The progressive accumulation of lactate in the myocardium during aortic clamping identified in biopsies, reinforces the idea of an anaerobic metabolism, at least in the region where the biopsies were performed. Lactate production continued to increase during the first ten minutes of reperfusion. This anaerobic metabolism in the presence of what probably is normal oxygen release can indicate the presence of temporary myocardial cell dysfunction [18]. After only 60 minutes of reperfusion the lactate production was reduced.
02 concentrations
The oxygen myocardial concentrations were very low after normothermic cardioplegic induction and after aortic declamping. This concentration increased progressively during reperfusion, although after 60 minutes of reperfusion it was still significantly lower than the pre-CPB control. This may reinforce the hypothesis that there is a reduction in the cellular capacity of using the supplied O2. This cellular metabolic dysfunction, of at least one group of cells, is probably at the mitochondrial plane [4, l8-20],
ATP reserves
In relation to the adenine nucleotide levels there was a preservation of these during aortic clamping, probably due to low consumption during asystole [21]. But, even so, there is an increase in the ADP and AMP levels because of the breakdown of the ATP. These metabolic parameters also indicate that there was an increase in the production of ATP 30 minutes after the start of reperfusion, even though the levels remained low. However, in spite of starting metabolic recovery, ATP aerobic production is not enough to maintain the ATP levels. This inadequate cellular metabolism during reperfusion might be due to mitochondrial alterations with transitory incapacity to maintain a normal aerobic metabolism. Also it is possible that depletion of adenine nucleotides with consequent loss of ATP precursors had delayed the regeneration of myocardial ATP [14, 19, 22-25].
CK-MB
Postoperative serum CK-MB levels observed in this group of patients (Figure 4) may indicate a temporarily compromise in cellular function. The cellular membrane is very sensitive to ischemia and after prolonged periods of anoxia even relatively big molecules such as enzymes can cross the cellular membrane. But, comparing the postoperative serum CK-MB curve of this group with the group of our earlier work [12] (Figure 4), in which normothermic anterograde induction was not utilized, an important reduction in the postoperative serum CK-MB levels was seen in this work. However, it seems that myocardial protection by continuous retrograde hypothermic sanguineous cardioplegia was improved with the addition of normothermic anterograde induction.
CONCLUSIONS
The method could not avoid anaerobic myocardial metabolism during aortic clamping, even though continuous sanguineous cardioplegia was used. Alterations in the myocardial cellular metabolism during the first hour of reperfusion were observed. Alterations in the cellular metabolism are probably transitory, as almost complete recovery occurs 60 minutes after the start of reperfusion.
Compared to the earlier work [16], the addition of normothermic induction reduced the time to obtain asystole (Table 1) and there was a significant reduction in the postoperative CK-MB serum levels (Figure 4).
The clinical results obtained were good and this was considered a safe and simple method. Further metabolic studies using different temperatures of cardioplegia and the use of ATP precursors are suggested.
ACKNOWLEGEMENTS
The authors wish to thank the nurses Laila Östersjö, Jenny Larsson, Catharina Hjelm and Jane Strand, for their dedication to the patients of this study, as well as, the anesthesiologists, perfusionists and nurses of the intensive care unit.
BIBLIOGRAPHIC REFERENCES
1. Mentzer Jr RM, Jahania MS, Lasley RD. Myocardial protection. In: Cohn LH, Edmunds Jr LH, eds. Cardiac surgery in the adult. New York:McGraw-Hill, 2003:413-38.
2. Åmark K, Berggren H, Björk K, Ekroth A, Ekroth R, Nilsson K et al. Blood cardioplegia provides superior protection in infant cardiac surgery. Ann Thorac Surg. 2005;80(3):989-94.
3. Catinella FP, Cunningham JN, Adams PX, Snively SL, Gross RI, Spencer FC. Myocardial protection with cold blood potassium cardioplegia during prolonged aortic cross-clamping. Ann Thorac Surg. 1982;33(3):228-33.
4. Bomfim V, Kaijser L, Bendz R, Sylvén C, Morillo F, Olin C. Myocardial protection during aortic valve replacement: cardiac metabolism and enzyme release following continuous blood cardioplegia. Scand J Thorac Cardiovasc Surg. 1981;15(2):141-7.
5. Khuri SF, Warner KG, Josa M, Butler M, Hayes A, Hanson R, Siouffi S et al. The superiority of continuous cold blood cardioplegia in the metabolic protection of the hipertrophied human heart. J Thorac Cardiovasc Surg. 1988;95(3):442-54.
6. Panos A, Christakis GT, Lichtenstein SV, Wittnich C, El-Dalati N, Salerno TA. Operation for acute postinfarction mitral insufficiency using continuous oxygenated blood cardioplegia. Ann Thorac Surg. 1989;48(6):816-9.
7. Louagie YA, Jamart J, Gonzalez M, Collard E, Broka S, Galanti L et al. Continuous cold blood cardioplegia improves myocardial protection: a prospective randomized study. Ann Thorac Surg. 2004;77(2):664-71.
8. Gundry SR, Kirsh MM. A comparison of retrograde cardioplegia versus anterograde cardioplegia in the presence of coronary artery obstruction. Ann Thorac Surg. 1984;38(2):124-7.
9. Partington MT, Acar C, Buckberg GD, Julia P, Kofsky ER, Bugyi HI. Studies of retrograde cardioplegia I. Capillary blood flow distribution to myocardium supplied by open and occluded arteries. J Thorac Cardiovasc Surg. 1989;97(4):605-12.
10. Bothe W. Retrograde administration. Multimidia manual of cardiothoracic surgery. (august 9, 2005) 809:711.
11. Borger MA, Rao V, Weisel RD, Floh AA, Cohen G, Feindel CM et al. Reoperative coronary bypass surgery: Effect of patent grafts and retrograde cardioplegia. J Thorac Cardiovasc Surg. 2001;121(1):83-90.
12. Mallidi HR, Sever J, Tamariz M, Singh S, Hanayama N, Christakis GT et al. The short-term and long-term effects of warm or tepid cardioplegia. J Thorac Cardiovasc Surg. 2003;125(3):711-20.
13. Yau TM, Weisel RD, Mickle DA, Ivanov J, Mohabeer MK, Tumiati L et al. Optimal delivery of blood cardioplegia. Circulation. 1991;84(5 Suppl): III 380-8.
14. Rosenkranz ER, Vinten-Johansen J, Buckberg GD, Okamoto F, Edwards H, Bugyi H. Benefits of normothermic induction of blood cardioplegia in energy-depleted hearts, with maintenance of arrest by multidose cold blood cardioplegic infusions. J Thorac Cardiovasc Surg. 1982;84(5):667-77.
15. Hanafy HM, Allen BS, Winkelmann JW, Ham J, Osimani D, Hartz RS. Warm blood cardioplegic induction: an underused modality. Ann Thorac Surg. 1994;58(6):1589-94.
16. Sobrosa C G, Jansson E, Kaijser L, Bomfim V. Metabolismo miocárdico após cardioplegia sanguínea hipotérmica retrógrada contínua. Rev Bras Cir Cardiovasc. 2000;15(3):219-26.
17. Magovern Jr GJ, Flaherty JT, Gott VL, Bulkley BH, Gardner TJ. Failure of blood cardioplegia to protect myocardium at lower temperatures. Circulation. 1982;66(2 pt 2):60-7.
18. Engelman RM, Rousou JH, Lemeshow S, Dobbs WA. The metabolic consequences of blood and crystalloid cardioplegia. Circulation. 1981;64(2 pt 2):II 67-74.
19. Rosenkranz ER, Okamoto F, Buckberg GD, Vinten-Johansen J, Allen BS, Leaf J et al. II- Biochemical studies: failure of tissue adenosine triphosphate levels to predict recovery of contractile function after controlled reperfusion. J Thorac Cardiovasc Surg. 1986;92(3 pt 2):488-501.
20. Bomfim V. Continuous blood cardioplegia: a unique approach. In: Engelman RM, Levitsk S, eds. A textbook of clinical cardioplegia. New York:Futura;1982. p.265-76.
21. Rosenkranz ER, Okamoto F, Buckberg GD, Vinten-Johansen J, Robertson JM, Bugyi H. Safety of prolonged aortic clamping with blood cardioplegia. II- Glutamate enrichment in energy-depleted hearts. J Thorac Cardiovasc Surg. 1984;88(3):402-10.
22. Ely SW, Mentzer RM Jr, Lasley RD, Lee BK, Berne RM. Functional and metabolic evidence of enhanced myocardial tolerance to ischemia and reperfusion with adenosine. J Thorac Cardiovasc Surg. 1985;90(4):549-56.
23. Bolling SF, Bies LE, Gallagher KP, Bove EL. Enhanced myocardial protection with adenosine. Ann Thorac Surg. 1989;47(6): 809 15.
24. Bolling SF, Bies LE, Bove EL, Gallagher KP. Augmenting intracellular adenosine improves myocardial recovery. J Thorac Cardiovasc Surg. 1990;99(3):469 74.
25. Fiore AC, Naunheim KS, Kaiser GC, Willman Vl, McBride LR, Pennington DG et al. Coronary sinus versus aortic root perfusion with blood cardioplegia in elective myocardial revascularization. Ann Thorac Surg. 1989;47(5):684-8.






 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license