INTRODUCTION
Implantation of biological prosthesis during heart valve replacement reduces the risk of thromboembolic events and avoids specific anticoagulation therapy [1]. The improvement in the quality of life is set against its durability, which is the reason for several changes in the preparation of biological tissues and prosthesis projects [2-5]. The concept of bioprosthesis, introduced by Carpentier et al. [6], allows favorable long-term results with heterologous prostheses manufactured from porcine aortic valves [7].
Bovine pericardial bioprostheses were introduced by Ionesco, aiming at improving the durability and hemodynamical performance compared to porcine aortic valve bioprostheses [8,9]. Initial tests in a pulse simulator identified the possibility of early structural failure [10], but changes in the project and in the technique of tissue preparation gave favorable hemodynamic and clinical results [11,12]. Their use in Brazil was publicized by Braile et al. [13] and the St Jude Medical-Biocor TM prosthesis (SJM-Biocor TM) was introduced in 1982 [14]. This model incorporates structural changes in the pericardial membrane that give a better durability and a satisfactory hemodynamical result [6].
The aim of the current study is to present the results observed in patients who underwent valve replacement with the implantation of SJM-Biocor TM bovine pericardial bioprostheses.
METHOD
Patients
From November 1992 to December 2000, 358 consecutive patients were submitted to the implantation of, at least one, SJM-Biocor TM bovine pericardial prosthesis. From these, 304 patients, who were discharged from the Institute of Cardiology of Rio Grande do Sul / Fundação Universitária de Cardiologia, were included in this study. One hundred and fifty-one (49.6%) patients were female and 153 (50.3%) were male. Ages ranged from 15 to 83 years with a mean and standard deviation of 60.6 ± 14.3 years. Thirty-five (11.5%) patients were under 40 years old, 82 (27.0%) between 40 and 60 years old and 187 (6l.5%) patients were over 60 years old.
The functional class according to the criteria of the New York Heart Association (NYHA) was II in 29.9%, III in 48.0% and IV in 22.0% of the cases.
The etiology of the valve injury was idiopathic in 125 (41.1%), rheumatic in 92 (30.3%), myxomatosis in 28 (9.2%), ischemic in 21 (6.9%), Marfan's syndrome in 11 (3.6 %), endocarditis in 11 (3.6%) and congenital in four patients (1.3%); 12 (4.0%) patients presented with dysfunction of bioprostheses.
From the total, 78 (25.6%) patients had been submitted to prior heart valve surgery. The total and mean follow-ups were 931.0 patient-years and 3.8 ± 2.0 patient-years, respectively.
Methods
Surgical procedures were performed according to the accepted routines, including the use of cardiopulmonary bypasses, myocardial preservation using hypothermic crystalloid cardioplegia and moderate hypothermia (from 32º to 28º C).
In respect to the site of implantation, 172 (56.6%) patients received isolated aortic valves, 109 (35.9%) isolated mitral valves, 20 (6.6%) mitral-aortic valves and three (1.0%) tricuspid valves.
Associated heart procedures occurred in 116 (38.1%) patients, including coronary artery bypass grafting surgery in 67 (22.0%), tricuspid repair in 16 (5.2%), mitral repair in nine (2.9%), repair of congenital defects in seven (2.3%) and the Cox-Maze surgery in one (0.3%).
After surgery the patients were treated in the recovery room (intensive care unit) for a minimum of 48 hours. Hospital discharge occurred after the 5th postoperative day and oral anticoagulation therapy was not routinely prescribed; this was indicated in cases of chronic atrial fibrillation, greatly enlarged left atria or in patients with mechanical valve prostheses. Prophylactic antibiotic therapy for bacterial endocarditis was used [15].
After hospital discharge, the patients were referred to an assistant physician or were followed up in the outpatients' clinic of the Institution. The clinical situation of each consultation was registered on the patient's record card, as were clinical or significant surgical events [16]. For patients from other institutions, direct contact was made with the referring physicians, aiming at obtaining the patient's postoperative history and after, up-to-date information on the patient.
This study was approved by Ethics Research Committee of the Institute of Cardiology of Rio Grande do Sul.
Events
Important events related to the bioprostheses included fibrocalcific degeneration, bioprosthetic thromboses with or without peripheral embolic events, peri-valve fistulae and infections (endocarditis, even without identifying the infectious agent).
Surgical events included redo heart surgeries due to failure of the bioprostheses or other reasons (such as replacement of another heart valve or coronary artery bypass grafting surgery).
Deaths were classified as attributable to the bioprosthesis (for example thrombosis, endocarditis or dysfunction resulting in heart failure implicating the surgery or not), caused by heart disease (arrhythmia or heart failure not related to the failure of the bioprosthesis) or of non-heart causes (due to a systemic disease such as neoplasia).
Data analysis
The late mortality, survival rate (for all patients and according to classification in demographic and operative characteristics such as age, gender, etiology of the injury and position of the valve implant), event-free survival (in which events related to the bioprosthesis were associated to deaths), the durability of the bioprosthesis (considering failure related to the bioprosthesis) and the probability of structural failure were considered. Clinical evolution, considering changes in the functional class according to the NYHA criteria, was also evaluated.
The data are shown in tables of contingence and statistically analyzed using the actuarial method [17] and the Student t-test, chi-square and Kaplan-Meier tests in the SPSS computer program. Quantitative values are expressed as means ± standard deviations. The accepted significance level, critical a, was 5% (p-value < 0.05). Aiming at stressing the long-term results of the bioprostheses being studied, the actuarial curves were set up without taking into account hospital morbimortality that is, from the moment of hospital discharge, as events in this period are predominantly caused by heart failure, not related to the bioprosthesis. To include the effect of immediate mortality in the long-term results, it is necessary to multiply the long-term survival, at any point on the curve, by a specific index (SI) for each subgroup that is assessed. This index results from the immediate mortality specific to each subgroup of patients. Thus, the immediate mortality of the entire sample studied (15.0%) gives a SI of 0.85. The SIs of the subgroups are 0.89 for aortic patients, 0.8 for mitral patients, 0.9 for under 40-year-olds, 0.83 for patients between 40 and 60 years and 0.85 for over 60-year-olds. The immediate mortality does not exert any influence on the durability curves, when considering events related to the bioprosthesis alone, as no events occurred before discharge from hospital.
RESULTS
Mortality and morbidity
The immediate mortality was 15.0% without any deaths related to bioprostheses. In the follow-up, 28 deaths occurred (3.0% per patient-year), with 5 (0.5% per patient-year) related to bioprostheses (endocarditis: two; CVA/thromboembolism: two; and bioprosthesis dysfunction: one), seven (0.7% per patient-year) due to other heart problems (heart failure: three; ventricular arrhythmia: three; acute myocardial infarction: one), four (0.4% per patient-year) due to non-heart diseases (neoplasia: three; sepsis: one) and 12 (1.2% per patient-year) of unknown causes.
Events related to the bioprostheses included endocarditis: 18 (1.9% per patient-year) episodes in 17 patients; fibrocalcific degeneration/rupture: 15 (1.6% per patient-year), with only one case of rupture; thromboembolism: three (0.3% per patient-year); hemolysis: one (0.1% per patient-year). One case of a fistula after valve replacement was reported.
The patients who suffered from fibrocalcific degeneration or rupture had a mean age of 46.5 ± 11.6 years; a mean age lower than the patients without this complication, 61.7 ± 17.8 years (p-value < 0.05). Bioprosthesis dysfunction resulted in 16 (5.2%) reoperations due to: fibrocalcific degeneration/rupture (nine), endocarditis (six) or thromboembolism (one). Of the reoperated patients, five received replacements using SJM Biocor TM bovine pericardial bioprosthesis, giving a total of 309 bioprostheses utilized.
Three (0.9%) patients were reoperated for valve replacement using mechanical prosthesis in positions different to the position of the bioprosthesis under study and eleven for the implantation of pacemakers.
Endocarditis was diagnosed in from 1 to 99 months after the operation (mean 27.8 months). Five patients presented endocarditis before the third postoperative month (27.7% of the episodes). The probable cause of infection was identified in five patients (27.7% of the episodes): infection of the urinary tract in three and one case each of oophorectomy and bronchopneumonia. The infectious agent was identified in seven patients: Enterococcus faecalis (two), Staphylococcus epidermis (two), negative Staphylococcus coagulase (one), Staphylococcus aureus (one) and Pseudomonas aeruginosa (one). Endocarditis infection resulted in two deaths, six reoperations, one patient continues to be treatment and nine patients responded favorably to the antibiotic therapy.
Postoperative survival
The probability of survival for all patients was 86.3 ± 3.4% at 5 years and 69.3 ± 9.0% at 10 years (Figure 1).
Probabilities of survival of 90.6 ± 4.2% and 81.2 ± 5.7% at 5 years and 65.7 ± 12.4% and 81.2 ± 5.7% (p-value < 0.05) at 10 years were observed for female and male patients respectively.
Under 40-year-olds demonstrated a survival rate of 94.8 ± 7.1% at 5 years and 82.0 ± 13.3% at 9 years; the age group between 40 and 60 years had survival rates of 85.5 ± 6.8% over both time intervals: and the over 60-year-old group demonstrated a survival of 85.6 ± 4.4% at 5 years and 58.8 ± 13.6% at 10 years (Figure 2).
In an investigation concerning the etiology of the valve disease, different survival rates were observed contributing to a lower number of patients in some of the groups. For the most severe heart diseases (at least 18 patients), the survival rates at 5 and 10 years were 83.6 ± 6.2% and 73.7 ± 10.7% respectively for idiopathic disease, 91.5 ± 5.0% and 65.5 ± 14.5% for rheumatic disease, 83.8 ± 11.1% at both time intervals for myxomatosis degeneration and 69.8 ± 13.9%, again at both time intervals, for ischemic disease.
Patients who underwent mitral valve replacement had lower probability of survival than patients who underwent aortic valve replacement at 5 years (81.7 ± 6.0% and 87.3 ± 4.8%) and 10 years (65.1 ± 10.7% and 74.8 ± 9.1%) - Figure 3.
Thus, according to the SI to evaluate the effect of hospital mortality, the results of survival at 5 and 10 years are respectively 73.3 ± 2.9% and 58.9 ± 7.6%, for the entire sample studied; 85.3 ± 6.5% and 73.8 ± 12.3% (at 9 years), for under 40-year-olds, 72.7 ± 3.8% and 49.9 ± 11.5% for over 60-year-olds, 65.3 ± 4.2% and 52.0 ± 8.0% for patients who underwent mitral valve replacement and 77.6 ± 4.8% and 66.5 ± 8.6% for those who underwent aortic valve replacement. The SI for patients between 40 and 60 was 70.9 ± 5.6% over both time intervals.
Event-free survival
The probability of event-free survival was 77.5 ± 3.7% at 5 years and 40.2 ± 9.0% at 10 years (Figure 1). According to age, the event-free survival of the youngest group (< 40 years) was 71.7 ± 10.1% at 5 years and 27.2 ± 17.1% at 9 years; for the group between 40 and 60 years it was 76.4 ± 7.4% at 5 years and 39.7 ± 18.1% at 10 years and for the over 60-year-old group it was 80.0 ± 4.6% at 5 years and 44.3 ± 11.8% at 10 years (Figure 4). In respect to the position of the bioprosthesis, for patients who were submitted to mitral valve replacement, the event-free survival rate was 73.6 ± 6.1% at 5 years and 38.3 ± 10.5% at 10 years, while for those who were submitted to aortic valve replacement it was 78.9 ± 5.4% and 56.3 ± 10.2% for 5 and 10 years respectively (Figure 5).
Considering hospital mortality, the event-free survival rates at 5 and 10 years were 65.8 ± 3.1% and 34.1 ± 7.6% respectively for the entire sample; 64.5 ± 9.0% and 24.4 ± 15.3% (at 9 years) for under 40-year-olds, 63.4 ± 6.1% and 32.9 ± 15.0% for patients between 40 and 60 years, 68.0 ± 3.9% and 37.6 ± 10.0% for over 60-year-olds; 58.8 ± 4.9% and 30.6 ± 8.4% for patients submitted to mitral valve replacement and 70.2 ± 4.8% and 50.1 ± 9.1% for patients submitted to aortic valve replacement.
Bioprosthesis durability
The estimation of the bioprosthesis durability was 87.8 ± 2.5% at 5 years and 57.7 ± 9.9% at 10 years (Figure 6). Considering the effect of age, the estimated durability to the implantations in the youngest patients (< 40 years) was 76.7 ± 8.5% at 5 years and 34.5 ± 20.7% at 9 years. For patients between 40 and 60 years and for those with ages greater than 60 years, the estimation was 83.2 ± 5.7% and 93.1 ± 2.2% and 43.2 ± 19.5% and 75.8 ± 9.7% for the corresponding time intervals (Figure 7).
The estimation of aortic bioprosthesis durability was 89.2 ± 3.5% at 5 years and 75.4 ± 9.4% at 10 years. For mitral valve bioprostheses, the durability was 85.4 ± 4.4% and 57.0 ± 11.7% respectively (Figure 8).
The probability of structural failure for all bioprostheses was 5% over 5 years and 20% over 10 years. For bioprosthesis in the aortic position, the probability of structural failure was zero and 8% over the same time intervals.
Change in the functional class
In recent follow-ups, 88.5% of the patients were in functional class I, 9.1% in class II and 2.3% in class III, demonstrating a significant improvement from the preoperative period (p-value < 0.05).
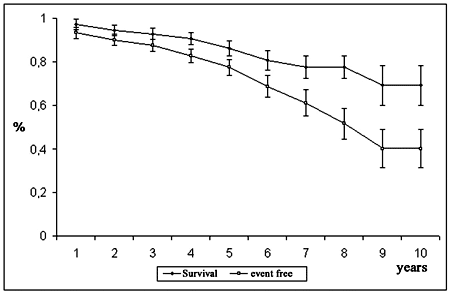
Fig. 1 - Probability of survival and event free survival
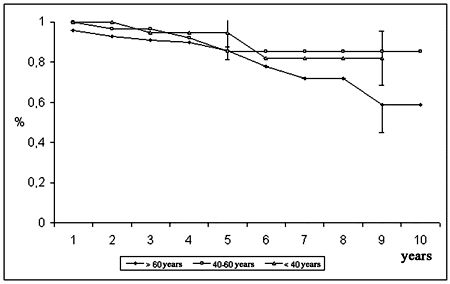
Fig. 2 - Probability of survival, according to age group of patients
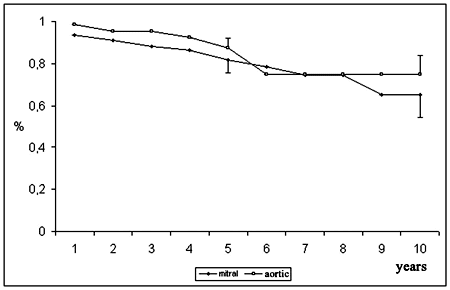
Fig 3 - Probability of survival, according to position of implantation
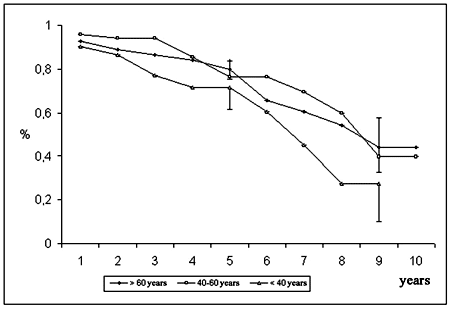
Fig. 4 - Event free survival according to age group of patients
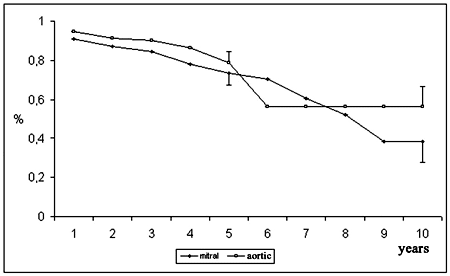
Fig. 5 - Event free survival, according to position of implantation
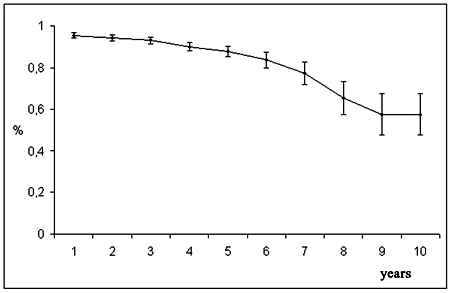
Fig. 6 - Durability of the bioprosthesis
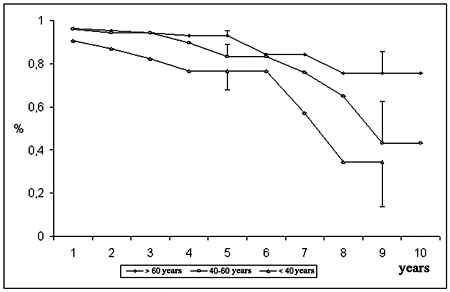
Fig. 7 - Durability of the bioprosthesis, according to age group of patients
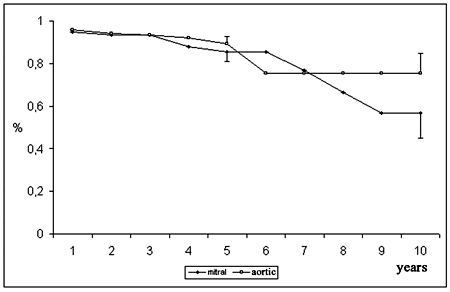
Fig. 8 - Durability of the bioprosthesis, according to position of implantation
Due to the large numbers of available replacement valves, the performance of the different prostheses over time is very important in the choice of the best valve for implantation. This study shows the results of the implantation of SJM-BiocorTM bovine pericardial bioprosthesis in patients with heart valve disease comparing them with the results in the literature [18-21].
As the main goal of the study is an analysis of the long-term survival of patients, as well as events related to the bioprosthesis, survival curves were calculated from hospital discharge, as the factors determining immediate mortality are mainly related to the clinical condition of the patient and to the surgical procedure, in detriment of the hemodynamic performance and possible complications of the bioprosthesis [22-26].
The mortality rate of 9.2% over 10 years of follow-up is acceptable, as there have been rates of up to 46% reported [19,20,27]. As many variables can contribute to late deaths or influence the absolute number of deaths (patients exposed to risks each year, follow-up time), the causes of death must be considered. Five deaths related to bioprostheses occurred (17.8% of the total), as a consequence of endocarditis, structural dysfunction and thromboembolism, a finding similar to a reported 22% [18]. In this series of patients, 56% of the late deaths were caused by heart problems, a number greater than in our investigation (25%). This difference may be justified by the high rate of deaths due to unknown causes in our series. It is relevant that failure of the prosthesis resulted in only a few deaths.
The probability of survival for all the patients in our series was 86.3 ± 3.4% at 5 years and 69.3 ± 9.0% at 10 years, while other authors reported rates of from 46% to 71% for this interval [18,20,21]. The estimation of event-free survival was low (40.2 ± 9.0% over 10 years), a fact which was also observed in patients with other types of bioprosthesis [28,29], and attributable to factors such as age of the patients, severity of the heart disease, position of the implanted prosthesis and postoperative prophylaxis for endocarditis and thromboembolism.
One factor to be considered is related to the age of the patients. For under 40-year-old patients, the probability of survival over 9 years was 82.0 ± 13.3%, while event-free survival was only 27.2 ± 17.1%. A significant difference between actuarial and event-free survival is expected in this population as patients in this age group present with a greater number of complications related to the bioprosthesis, particularly fibrocalcific degeneration [30,31].
The satisfactory expectation of survival can be explained by an effective neutralization of complications (such as low mortality in reoperations for valve dysfunction and curative clinical treatment of endocarditis). A similar result was obtained with patients of the intermediary age group: actuarial survival of 85.5 ± 6.8% both at 5 and 10 years and event-free survival of 76.4 ± 7.4% and 39.7 ± 18.1%, respectively. The survival of over 60-year-old patients was 85.6 ± 4.4% at 5 years and 58.8 ± 13.6% over 9 years, percentages that are partially justified by the advanced age and by the terminal repercussions of heart disease (and systemic diseases). Event-free survival was similar to the actuarial survival in both time intervals (80.0 ± 4.6% and 44.4 ± 11.8%, respectively), showing few non-fatal events. These findings endorse the use of bioprosthesis in elderly patients [20,21,31].
The probability of survival in respect to subjacent heart disease was variable (Figure 4), with more favorable results in patients with myxomatous and idiopathic heart diseases. The reduced number of patients in some groups hinders a clear understanding of the effect of subjacent heart diseases on actuarial and event-free survival.
The estimation of survival in relation to the position of the bioprosthesis has been the subject of many studies. Our results for patients who were submitted to aortic valve replacement show a survival rate of 74.8 ± 9.1% at 10 years. Patients with 2nd-generation Carpentier-Edwards bovine pericardial bioprostheses present with very similar expected survival rates, with values of from 40% to 70%, over 10 years [21,22,31] and from 34% to 53% over 12 years of follow-up [19,20,32]; some studies with lower estimations include operative mortality. In our series, patients who underwent mitral valve replacement had a lower survival rate when compared with patients after aortic valve replacement, 65.1 ± 10.7% at 10 years. Publications report expected survival rates of between 62% to 73% at 8 years, 57% over 10 years, 54% in the 12th and 37% in the 15th year [18,21,31,32].
For the period of 10 years, the follow-up time of our study, SJM-BiocorTM bioprostheses maintained a performance similar to Carpentier-Edwards bioprostheses both in the aortic and mitral positions and we conjecture results comparable over longer follow-up times, but this needs to be confirmed with future investigations.
In respect to morbidity, the prevalence of endocarditis was 5.9% and resulted in six reoperations (38% of the explanted bioprosthesis) and two deaths (7.1% of the total deaths). In spite of being recognized as a common cause of death [18,20], bioprosthesis dysfunction (prevalence from 4.2% to 6.8%) [20,21,27] or reoperation (responsible from 6.3% to 13% of the explanted bioprosthesis) [20,27,33], the prevalence of endocarditis should be considered as high. Taking into consideration the patients exposed to risk, the literature shows an incidence of endocarditis of from 0.4% to 2.4% per patient-year [13,34], while in our series we observed a rate of 1.9% per patient-year.
The mean time between bioprosthesis implantation and the start of endocarditis was 25 months (ranging from 2 to 99 months). The occurrence of four infections within the first 3 postoperative months and the identification of a probable entry point in five patients are significant. If a second episode of endocarditis in one patient, 2 months after the first, were included in this last observation, more than 50% of the cases would have the cause defined as not related to the prosthesis. However, patients must be counseled on prophylactic antibiotic treatment. Also, a consequent response should be expected if endocarditis were suspected (or confirmed by blood cultures).
Structural failure resulted in one death and nine reoperations; as 15 patients presented with this complication, six patients with diagnoses of prosthetic failure remained under clinical observation waiting for reoperations. When diagnosis is established, the urgency for reoperation depends on the clinical condition of the patient, on the degree of failure and on the characteristics of the patient.
Structural failure is an important determining factor of the performance of the bioprosthesis. The durability of the bioprostheses was estimated at 75.4 ± 9.4% over 10 years in the aortic position and 57.0 ± 11.7% in the mitral position. In the literature, for patients who undergo aortic valve replacement, the estimated freedom from structural deterioration, equivalent to durability, has been reported as being between 91% and 100% over 10 years and 82% and 100% over 12 years [19,20,32,35]. For Carpentier-Edwards mitral bioprostheses the values were 76% over 10 years [18] and 78% over 12 years [36]. The age affects the durability of the bioprosthesis, as younger patients present lower values than the older population [33]. The probability of structural failure for all bioprosthesis was 5% over 5 years and 20% over 10 years. For aortic bioprosthesis the results were better, with an estimation of zero and 8% for the same time intervals. Even so the performance of the aortic bioprosthesis is highly satisfactory.
Prosthesis failure due to fibrocalcific degeneration or rupture is a serious concern when bioprosthesis implantation is considered. These events are mainly related to the quality and to the treatment of the biological tissue [6,34] and with the age of the patient [21,25]. rupture specifically refers to the design of the bioprosthesis and its assembling [6,10]. As in the production of the current generation of bioprosthesis, including the SJM-BiocorTM, special attention is paid to the hemodynamic performance and to the mechanical durability, as well as to the project and assembling of bovine pericardial membrane (including the internal lining); failures due to the project and to assembling of bioprostheses are few. Structural deterioration is mainly related to the age of the patient and evidence of low incidences or even no fibrocalcific degeneration in older populations has been reported in publications [18,20,31].
The mean age of patients with fibrocalcific degeneration was significantly lower than the mean age of the patients without this complication, showing that this complication is more common in younger individuals. The prevalence of structural dysfunction was 4.9% in our series, lower than the 1.7% seen in a series of patients who underwent aortic and mitral valve replacements followed up over a period of 12 years [29], but comparable with individual series of patients submitted to mitral (from 2.1% to 2.7%) and aortic valve (up to 8.2%) replacements [19,20]. For a follow-up period of up to 15 years, cases of dysfunction increased to 12.4% for mitral bioprosthesis implantation [33].
Thromboembolism was identified in three (0.9%) patients and was a possible cause of death in two others who suffered cerebrovascular ischemia. Another patient had to be submitted to a reoperation for mitral bioprosthesis replacement due to thrombosis both of the atrium and bioprosthesis. These events, also observed in other series [18,20,21,33], show that some patients undergoing valve replacement with bioprostheses, additional to those with recognized indications, which include patients with chronic atrial fibrillation, enlarged left atrium or concomitant mechanical prosthesis, must receive prophylactic anticoagulation,. There are studies of patients with pericardial bioprosthesis that demonstrate a similar number of events caused by bleeding complications related to anticoagulant therapy and episodes of thromboembolism [20]. Because of this, we take special care to consider the evolution of individual patients before indicating oral anticoagulation.
Combining our results with published results, it is obvious that the main indication of bioprosthesis implantation is to replace aortic valves in over 60-year-old patients, as the risk of prosthesis failure is low [1,20,27]. The results of elderly patients who undergo mitral valve replacement are also expected to be good [18,33,34]. Young patients (under 40-year-olds) present a high probability of failure over a long term with the necessity of reoperations. The implantation of a bioprosthesis must be indicated if a mechanical apparatus can not be utilized, due to intellectual, social or economical reasons. Women who wish to become pregnant also require the implantation of a bioprosthesis and in these circumstances our results indicate that the SJM-BiocorTM may be considered.
The clinical condition of patients is an indirect measurement of the hemodynamical performance of bioprostheses. It was observed that 97.6% of the patients with recent follow-ups are in functional classes I or II (NYHA), a result similar to those described in the literature [27,29,32,36]. The hemodynamical performance of SJM-BiocorTM bioprostheses was the subject of pulse simulating tests demonstrating an adequate result [6]. It is important to emphasize that in this series there were no cases of valve replacement due to flow obstruction (stenosis) with normally functioning bioprostheses.
CONCLUSION
This study presents some limitations, such as the small sample size of subgroups (mainly in groups with an etiology of heart disease and in the younger age group) and with the 10 years of complete follow-up. However, our results are comparable to findings reported in other publications on 2nd-generation bovine pericardial bioprosthesis available on the market and guarantee the quality of SJM-BiocorTM bioprostheses for heart valve replacement.
ACKNOWLEDGMENT
The current study was partially supported with help offered to Dr. João Ricardo Michielin Sant'Anna by the Brazilian National Research Counsel (CNPq).
BIBLIOGRAPHIC REFERENCES
1. Birkmeyer NJ, Birkmeyer JD, Tosteson AN, Grunkemeier GL, Marrin CA, O`Connor GT. Prosthetic valve type for patients undergoing aortic valve replacement: a decision analysis. Ann Thorac Surg. 2000;70(6):1946-52.
2. Wallace RB. Tissue valves. Am J Cardiol. 1975;35(6):866-71.
3. Jones EL, Craver JM, Morris DC, King III SB, Douglas Jr JS, Franch RH et al. Hemodynamic and clinical evaluation of the Hancock xenograft bioprosthesis for aortic valve replacement (with emphasis on management of the small aortic root). J Thorac Cardiovasc Surg. 1978;75(2):300-8.
4. Fantini FA, Vrandecic MO, Gontijo Filho B, Oliveira OC, Martins Jr IC, Marinho AA et al. Biopróteses aórticas porcinas, modelo convencional e sem suporte ("stentless"): estudo comparativo. Rev Bras Cir Cardiovasc. 1998;13(3):221-8.
5. Pomerantzeff PMA, Brandão CMA, Braile DM, Albuquerque JMAC, Ramirez VDA, Camim A et al. Novo conceito de bioprótese: bioprótese com descontinuidade do anel de sustentação (less stented)®. Rev Bras Cir Cardiovasc. 2004;19(3):267-73.
6. Carpentier A, Dubost C, Lane E, Nashef A, Carpentier S, Relland J et al. Continuing improvements in valvular bioprostheses. J Thorac Cardiovasc Surg. 1982;83(1):27-42.
7. Rossiter SJ, Miller DC, Stinson EB, Oyer PE, Reitz BA, Moreno-Cabral RJ et al. Hemodynamic and clinical comparison of the Hancock modified orifice and standard orifice bioprostheses in the aortic position. J Thorac Cardiovasc Surg. 1980;80(1):54-60.
8. Ionescu MI, Tandon AP, Mary DA, Abid A. Heart valve replacement with the Ionescu-Shiley pericardial xenograft. J Thorac Cardiovasc Surg. 1977;73(1):31-42.
9. Braile DM, Volpe MA, Ramin SL, Souza DRS. Tratamento cirúrgico das valvopatias. Parte 1. Rev Bras Cir Cardiovasc. 1994;9(2):113-22.
10. Gabay S, Bortulotti U, Wasserman F, Factor SM. Hemodynamics and durability of mitral bioprostheses: an in vitro study. Eur Heart J. 1984;5(Suppl D):65-71.
11. Jamieson WR, Pelletier LC, Janusz MT, Chaitman BR, Tyers FO, Miyagishima RT. Five-year evaluation of the Carpentier-Edwards porcine bioprosthesis. J Thorac Cardiovasc Surg. 1984;88(3):324-33.
12. Grunkemeier GL, Bodnar E. comparative assessment of bioprosthesis durability in the aortic position. J Heart Valve Dis. 1995;4(1):49-55.
13. Braile DM, Ardito RV, Greco OT, Lorga AM. IMC Bovine pericardial valve: 11 years. J Card Surg. 1991;6(4 suppl):580-8.
14. Vrandecic MO, Gontijo BF, Silva JAP, Fantini FA, Barbosa JT. Estudo multicêntrico dos resultados das trocas valvares com o uso da bioprótese Biocor no Estado de Minas Gerais. Rev Bras Cir Cardiovasc. 1988;3(3):159-68.
15. Bonow RO, Carabello B, de Leon Jr AC, Edmunds Jr LH, Fedderly BJ, Freed MD et al. Guidelines for the management of patients with valvular heart disease: executive summary. A Report of the American College of Cardiology/American Heart Association Task Force on Pratice Guidelines (Committee on Management of Patients with Valvular Heart Disease). Circulation. 1998;98(18):1949-84.
16. Edmunds Jr LH, Clark RE, Cohn LH, Grunkemeier GL, Miller DC, Weisel RD. Guidelines for reporting morbidity and mortality after cardiac valvular operations. The American Association for Thoracic Surgery, Ad Hoc Liaison Committee for Standardizing Definitions of Prosthetic Heart Valve Morbidity. Ann Thorac Surg. 1996;62(3):932-5.
17. Grunkemeier GL, Jamieson WR, Miller DC, Starr A. Actuarial vs actual risk of porcine structural valve deterioration. J Thorac Cardiovasc Surg. 1994;108(4):709-18.
18. Aupart MR, Neville PH, Hammami S, Sirinelli AL, Meurisse YA, Marchand MA. Carpentier-Edwards pericardial valves in the mitral position: ten-year follow-up. J Thorac Cardiovasc Surg. 1997;113(3):492-8.
19. Dellgren G, David TE, Raanani E, Armstrong S, Ivanov J, Rakowski H. Late Hemodynamic and clinical outcomes of aortic valve replacement with the Carpentier-Edwards Perimount pericardial bioprosthesis. J Thorac Cardiovasc Surg. 2002;124(1):146-54.
20. Banbury MC, Cosgrove III DM, Lytle BW, Smedira NG, Sabik JF, Saunders CR. Long-term results of the Carpentier-Edwards pericardial aortic valve: a 12-year follow-up. Ann Thorac Surg. 1998;66(6 suppl):S73-6.
21. Pelletier LC, Carrier M, Leclerc Y, Dyrda I. The Carpentier-Edwards pericardial bioprosthesis: clinical experience with 600 patients. Ann Thorac Surg. 1995;60(2 suppl):S297-302.
22. Hannan EL, Racz MJ, Jones RH, Gold JP, Ryan TJ, Hafner JP et al. Predictors of mortality for patients undergoing cardiac valve replacement in New York State. Ann Thorac Surg. 2000;70(4):1212-8.
23. Ambler G, Omar RZ, Royson P, Kinsman R, Keogh BE, Taylor KM. Generic, simple risk stratification model for heart valve surgery. Circulation. 2005;112(2):224-31.
24. Jamieson WR, Edwards FH, Schwartz M, Bero JW, Clark RE, Grover FL. Risk stratification for cardiac valve replacement. National Cardiac Surgery Database. Database Committee of the Society of Thoracic Surgeons. Ann Thorac Surg. 1999; 67(4):943-51.
25. Jin R, Grunkemeier GL, Starr A. Validation and refinement of mortality risk models for heart valve surgery. Providence Health System Cardiovascular Study Group. Ann Thorac Surg. 2005;80(2):471-9.
26. Edwards MB, Taylor KM. Is 30-day mortality an adequate outcome statistic for patients considering heart valve replacement? Ann Thorac Surg. 2003;76(2):482-6.
27. Perier P, Mihaileanu S, Fabiani JN, Deloche A, Chauvaud S, Jindani A et al. Long-term evaluation of the Carpentier-Edwards pericardial valve in the aortic position. J Card Surg. 1991;64(4 suppl):589-94
28. Cohn LH, Collins Jr JJ, Rizzo RJ, Adams DH, Couper GS, Aranki SF. Twenty-year follow-up with the Hancock porcine xenograft in the elderly. Ann Thorac Surg. 1998;66(6Suppl):S35-9.
29. Grunkemeier GL, Bodnar E. Comparison of structural valve failure among different 'models' of homograft valves. J Heart Valve Dis. 1994;3(3):556-60.
30. Cosgrove DM, Lytle BW, Taylor PC, Camcho MT, Stewart RW, McCarthy PM et al. The Carpentier-Edwards pericardial aortic valve: ten-year results. J Thorac Cardiovasc Surg. 1995;110(3):651-62.
31. Aupart MR, Dreyfus XB, Meurisse YA, Rouchet SC, Sirinelli AL, May MD et al. The influence of age on valve related events with Carpentier-Edwards pericardial valves. J Cardiovasc Surg. 1995;36(4):297-302.
32. Neville PH, Aupart MR, Diemont FF, Sirinelli AL, Lemoine EM, Marchand MA. Carpentier-Edwards pericardial bioprosthesis in aortic or mitral position: a 12-year experience. Ann Thorac Surg. 1998;66(6 suppl):S143-47.
33. Marchand MA, Aupart MR, Norton R, Goldsmith IRA, Pelletier LC, Pellerin M et al. Fifteen-year experience with the mitral Carpentier-Edwards PERIMOUNT pericardial bioprosthesis. Ann Thorac Surg. 2001;71(5 suppl):S236-9.
34. Carpentier A, Lemaigre G, Robert L, Carpentier S, Dubost C. Biological factors affecting long-term results of valvular heterografts. J Thorac Cardiovasc Surg. 1969;58(4):467-83.
35. Poirer NC, Pelletier LC, Pellerin M, Carrier M. 15-year experience with the Carpentier-Edwards pericardial bioprosthesis. Ann Thorac Surg. 1988;66(6 Suppl):S57-61.
36. Dellgren G, David TE, Raanani E, Armstrong S, Ivanov J, Rakowski H. Late hemodynamic and clinical outcomes of aortic valve replacement with the Carpentier-Edwards Perimount pericardial bioprosthesis. J Thorac Cardiovasc Surg. 2002;124(1):146-54.








 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license