INTRODUCTION
The first attempts of oxygenating blood outside the body were made by physiologists in the XIX century in their studies on perfusion of organs isolated from animals.
The first modern studies dedicated to artificial oxygenation of blood, with the objective of maintaining the life of an organism, dated from 1937 and were performed by John Gibbon [1]. In the years that followed, Gibbon dedicated his research to the improvement of this revolutionary technique [2-4]. Several researchers, encouraged by the possibility of building apparatuses capable of replacing cardiopulmonary functions, started to build oxygenators [5-7].
Oxygenators must be capable of oxygenating approximately five liters of blood per minute and to remove the carbon dioxide (CO2) in order to artificially support the life of an adult. Moreover, heat exchange must be optimized in the least possible surface area, whilst the trauma caused to blood elements must be minimal. The priming necessary to operate the apparatus must also be small, in order to allow its use with acellular solutions, avoiding excessive hemodilution and unnecessary transfusions of blood or blood derivatives [8].
Several forms of supplying oxygen (O2) to the blood have been attempted, with more or less success, enabling the development of many models of oxygenators of which only a few have been clinically employed.
According to Gibbon [4], the oxygenator should produce a thin layer of blood that is in contact with O2 which he reported would be possible utilizing centrifugal force, forming bubbles of blood or running the blood over a screen. He also described several problems of artificial oxygenation including the formation of froth, hemolysis and the production of vasoactive substances.
Initial studies with membranes for gas exchange showed relative inefficiency of oxygenation for a blood flow sufficient to maintain patients on cardiocirculatory arrest [8]. When it became evident that the heart-lung machine could use low blood flows, according to the "azygos principle" popularized by Lillehei's group, researchers returned to study membranes, as a form of producing a more physiological artificial oxygenation, due to its similarity to the oxygenation achieved in the lungs [9]. These studies produced the first membrane oxygenators [10].
ORIGIN OF MEMBRANE OXYGENATORS
The first studies of artificial oxygenation of blood were thwarted by the formation of bubbles and froth, which were difficult to remove. Some researchers tried to utilize gas permeable membranes to make a physical separation between the blood and gas. Hence, the formation of bubbles was avoided and the artificial oxygenation became more similar to that that occurs in the lungs [8].
Kolff & Berk [11] in 1944 observed the arterialization of blood on crossing cellophane chambers in their first artificial kidney and started a series of studies that aimed at the utilization of the material as a gas exchange membrane. Barrer [12] and Van Amerongen [13] in 1948 demonstrated that some natural or synthetic elastomers were also permeable to gases.
The initial studies demonstrated that the choice of the material for the membrane is critical, because it must be compatible with blood, permeable to O2 and CO2, must be very thin with minimal resistance to blood and respiratory gas flow. These factors made the development of membrane oxygenators difficult. Kolff [14] in 1955 built the first prototype of a membrane oxygenator using polyethylene sheets which was successfully used in the experimental laboratory. Membranes were rolled around a central axe, giving the oxygenator the format of a reel.
Other materials such as cellophane, silicon and Teflon were utilized in the manufacture of different membrane oxygenators. Clowes & Neville [15] were pioneers in using a membrane oxygenator in heart surgery and in 1958 published a series of cases operated on using their apparatus. Their membranes were flat, produced from Teflon and set up with overlapping layers. The oxygenator was large, difficult to mount and frequently presented with leaks. Later, other membrane oxygenators with similar configurations to the original project of Clowes & Neville [16] were used [5,17,18].
Kolobow et al. [19], based on the Kolff model, projected an oxygenator composed of long strips of silicon sustained by an envelope with spacers to stop the membranes from sticking together. In this model, the blood flowed inside the strips and O2 circulated parallel to the central axis that sustained the reel of membranes. As this model functioned adequately for long periods, it was utilized in prolonged ventilatory support procedures. The Kolobow oxygenator was continuously improved and is currently produced and sold by the American Company, Avecor. It is the only oxygenator on the market recommended for prolonged use.
The first-generation membrane oxygenators had great resistance to the passage of blood through the membranes which was impossible to overcome by siphoning alone. Some apparatuses were set up on the side of the positive pressure of a peristaltic pump, whilst others required an additional pump to circulate the blood in the membrane chamber. Because of the difficulties with gas exchanges and the complexity of their construction and use, the first-generation membrane oxygenators were not very well accepted. The development of technology to produce expanded and capillary membranes made the appearance of the current generation oxygenators possible. In these membrane oxygenators, gas exchange occurs by diffusion of respiratory gases through a membrane permeable to gases, which are located between the blood and gas flows of the oxygenator, without direct contact between blood and gas [8].
These membrane oxygenators enable the independent control of the transference of O2 and CO2, using a gas mixer. The transference of O2 is controlled by the percentage of this gas instilled in the oxygenator. The higher the O2 fraction in the gas, the higher its transference to the blood will be. The transference of CO2 is controlled by variations in the flow of gas that ventilates the oxygenator. The higher the flow of gas, the higher the removal of CO2 of the blood will be.
The first reusable membrane oxygenator appeared in 1956, based on the principles of gas exchange in hemodialyzers in use at that time. The first disposable membrane oxygenators appeared in the 1960s. They were complicated to set up and to operate; they required an additional pump for the recirculation of the blood in the oxygenator aiming at improving oxygenation. This generation of oxygenators, according to as has already been mentioned, were not very popular [8].
GAS EXCHANGE PRINCIPLES
The transference of gases to the blood or to the gas that ventilates the oxygenator occurs due to diffusion, which is understood as the capacity of the molecules of the gas to move from an area of higher concentration to an area of lower concentration to reach a balance. The diffusibility or capacity of a gas to spread is constant for each gas, the material to be crossed and the temperature. The kinetic theory of gases demonstrates that diffusion depends on the velocity of the molecules of the gas and, according to Graham's law, it is inversely proportional to the square root of the molecular weight of the gas. Thus, the lower the molecular weight of the gas, the greater is its diffusibility. The velocity of the diffusion of a gas also increases proportionally to its solubility.
The diffusion and the transference of gases in membrane oxygenators are more complex than the laws on gases suggest, because there are several barriers to overcome. O2 must pass the membrane of oxygenators, be dissolved in the plasma of the blood, enter the membrane of red blood cells and spread in the cytoplasm of red blood cells to bind with the hemoglobin. The diffusion of CO2 from the blood is simpler than the diffusion of O2 because CO2 is approximately twenty times more soluble than oxygen and may be eliminated with great facility, simply by the partial pressure difference, through any type of membrane [8].
The diffusion of these gases inside the oxygenator depends on the type of material of the membrane, on its thickness and porosity, but it is also influenced by the thickness of the layer of blood in contact with the membrane and by the characteristics of the blood flow. The force that impels the gas through the membrane is denominated partial pressure. The higher the difference between the partial pressures, the higher the force that impels the gas will be. The velocity of the diffusion can also be increased reducing the distance the gas moves. In the case of oxygenators, this increased speed can be achieved reducing the thickness of the membrane.
Others factors, such as the type of blood flow, can accentuate the diffusion of gases through membranes. One resource frequently used during the construction of oxygenators is the production of secondary or "marginal" currents. These currents correspond to a certain swirling at the contact surface of the membrane with the blood, thereby favoring gas exchange. The existence of this turbulence may compensate the relative thickness of the layer of blood that passes through the oxygenator.
In a mixture of gases occupying a determined volume, each gas behaves as if it occupies all the volume of the mixture by itself, independent of the others gases. This, in essence, constitutes Dalton's law that governs the behavior of gases. The sum of the partial pressures of all gases in a mixture corresponds to the total pressure exerted by the mixture. These rules are applied to gases occupying a space or even in solution, as is the case of gases dissolved in blood. Thus, when the blood is in balance with the atmospheric gases, the sum of the partial pressures of gases dissolved in the blood will be 760 mmHg, at sea level. In the gaseous phase, the partial pressure, the concentration and the molecular fraction are extremely important.
In blood, O2 and CO2 do not behaved linearly as would be expected. This is due to the non-linearity of the disassociation of oxy-hemoglobin. This phenomenon is represented as a sinusoidal curve. Thus, CO2 binds to several substances of the plasma and red blood cells to form bicarbonate. All this means that O2 and CO2 exist in greater concentrations than would be expected by simple physical dissolution. Hence analysis of gas transference in oxygenators is much more complex.
TRUE AND MICROPOROUS MEMBRANES
The aforementioned general principles apply to all types of membrane oxygenators. In microporous membranes, the gases do not dissolve in the material of the membrane; on the contrary, they pass through microscopic pores that exist in the membrane. All the other variables that influence gas exchange also apply to microporous membranes.
Membrane oxygenators were conceived to function without the gas-blood interphase existent in other types of oxygenators. Membranes considered "true" constitute a barrier between the blood and gas, so that gas transference depends on diffusion in the material of the membrane. Gas, in truth, dissolves in the membranes and is released at the opposite surface, depending on the existing partial pressure gradient between the two sides of the membrane (Figure 1).
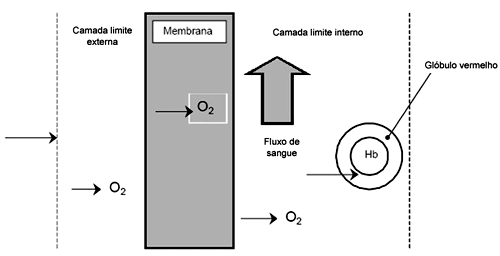
Fig. 1 - Diagram of oxygen diffusion through a porous membrane
True membrane oxygenators are expensive, difficult to produce, frequently require large areas of membrane and a large volume for priming. These oxygenators can maintain gas exchange of O2 and CO2 in satisfactory conditions for long periods (even weeks), without losing efficiency. These apparatuses are utilized in long-lasting procedures, such as ventilatory or prolonged circulatory support.
Microporous membranes, at least at the start of perfusion, allow a transitory interface between gas and blood. After some time a proteic lining is built up on the membrane which isolates the blood from the gas, but permits diffusion through micropores. The surface tension of the blood impedes leaking through the tiny pores of the capillary membrane. The micropores, in truth, function as ducts through a polypropylene membrane, providing the sufficient capacity of diffusion to both gases, O2 and CO2.
After some hours of use, the functional capacity of microporous membrane oxygenators is reduced due to evaporation and subsequent condensation of serum that passes through the micropores. There is evidence that heating the membranes and gas may delay this phenomenon and increase the durability of the apparatus.
The diameter of the pores of microporous membranes is smaller than 1 micron, although the exact size depends on the manufacturing process. It is always necessary that the micropores have less than 1 micron to inhibit leakage of liquid or gas through the surface of the membranes. Typically, during manufacture, the hollow fibers of polypropylene are extruded, ringed and elongated, to produce the micropores. After reaching the desired size, the fibers are heated to stabilize the structure of the polymer.
TYPES OF MEMBRANE OXYGENATORS
Membrane oxygenators in current use utilize microporous, silicon or polypropylene membranes. Other materials, such as Teflon and polyethylene were abandoned. Membrane oxygenators can be grouped in three principal types:
1. Plaque oxygenators - These are built with microporous membranes of expanded polypropylene, folded in a Z-shape, similar to the bellows of an accordion. In these apparatuses, blood and gas flow in opposite sides of the membrane. The main examples are Cobe Excel, Cobe VPCML, Shirley M-2000.
2. Spiral Oxygenators - These are a derivative of the old model of the Kobolov's oxygenator that utilized silicon membranes. The membrane is rolled around a central axis similar to a slightly-spiraled ball of yarn. There is only one apparatus of this type of oxygenator which is produced by the Company, Avecor.
3. Hollow fiber oxygenators - These are manufactured with microporous polypropylene membranes, constituted by capillary fibers positioned in parallel bunches or as skeins, forming hollow, capillary fibers or capillary membranes. This is the most common type the oxygenator used currently. These oxygenators are divided into two subgroups, according to the type of blood circulation.
A: Blood flows inside the capillary - In this model, blood circulates inside the capillary fiber bunches and the gas circulates outside, in opposite directions. There is a pressure gradient produced by the resistance of fibers on the passage of the blood and occasionally, thrombosis of a great number of fibers can occur, compromising the functioning of the oxygenator. The main examples are the Bentley CM40 and Terumo Capiox Serie 300.
B: Gas flows inside the capillary - In this model, gas passes through the capillary fiber bunches which are immersed in blood. This configuration reduces the gradient between blood and gas and, consequently, considerably reduces the trauma produced by the passage of blood inside the capillaries. This method also allows a reduction in the area of membrane necessary, optimizing the utilization of the dynamic characteristics of the apparatus. These are the most modern models and are replacing the others.
In these oxygenators, the venous blood may be preserved in a collapsible reservoir, such as a plastic bag, forming the concept of a "closed" circuit. These reservoirs frequently require the addition of an extra reservoir to receive the blood from suction called cardiotomy reservoirs to complete the perfusion circuit. Older models used collapsible reservoirs and have been replaced by rigid reservoirs with the functions of venous and cardiotomy reservoirs. Many of them incorporate a heat exchanger and the result is called an "open" circuit, very similar to the traditional circuit of bubble oxygenators. This configuration is preferred by the majority of the surgical and perfusion teams.
Many oxygenators available on the market offer two options of reservoirs, which are rigid or collapsible. The latest generation of devices is composed of a rigid reservoir connected to the membrane chamber and the heat exchanger as a single unit. These apparatuses are denominated as "integrated". In all the described models, the blood of the patient is collected in the venous reservoir, passes through the arterial pump, which impels it to the membrane oxygenator chamber. After gas exchange, blood flows through the arterial line to the arterial system of the patient.
NEONATE OXYGENATORS
The heart surgery of the newborn has been progressively increasing over the last years. Approximately 500 surgeries are performed each year in Brazil for the correction of complex congenial heart diseases [20]. As the newborn heart presents structural, functional and metabolic differences compared to adult hearts, a new generation of devices started to be developed. These new apparatuses have contributed to a significant reduction in the mortality associated to cardiopulmonary bypass (CPB) use in newborns. The high incidence of mortality is due to technical difficulties imposed by correction of some heart diseases and, principally, by the management of CPB with the small volumes filling the circuit [21]. The CPB procedures for the newborn require special apparatuses, due to the small margin of tolerance which these patients have to deviations in the physiology that affect the renal, hepatic, neurological, pulmonary and cardiovascular functions [22]. The majority of the oxygenators and other apparatuses destined for infant perfusion correspond to miniatures of the apparatuses developed for adults. There are few products developed specifically for the needs of the newborn. One of the difficulties is the utilization of volumes of perfusates which correspond to nearly three times the volemia (volume of blood) of the patient (on average, the volume of blood of volemia is 250 mL). The new generation of membrane oxygenators is specially built for neonatal perfusion and it has enabled a dramatic reduction of the volume of the perfusate. Braile et al. have dedicated themselves to the development and application of apparatuses utilized in heart surgery with CPB and specifically to membrane oxygenators [23-26].
BIBLIOGRAPHIC REFERENCES
1. Gibbon Jr. JH. Artificial maintenance of circulation during experimental occlusion of pulmonary artery. Arch Surg. 1937;34:1105.
2. Gibbon Jr. JH. The maintenance of life during experimental occlusion of the pulmonary artery followed by survival. Surg Gynecol Obstet. 1939;69:602.
3. Gibbon Jr. JH. An oxygenator with a large surface-volume ratio. J Lab Clin Med. 1939;24:1192.
4. Gibbon Jr. JH. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171.
5. Bramson ML, Osborn JJ, Gerbode F. The membrane lung. In: Ionescu MI, ed. Techniques in extracorporeal circulation. 2nd ed. London:Butterworth;1981.
6. Bramson ML, Hill JD, Osborn JJ, Gerbode F. Partial veno-arterial perfusion with membrane oxygenation and diastolic augmentation. Trans Am Soc Artif Intern Organs. 1969;15:412-6.
7. Gerbode F, Osborn JJ, Bramson ML. Experiences in the development of a membrane heart-lung machine. Am J Surg. 1967;114(1):16-23.
8. Souza MHL, Elias DO. Circulação extracorpórea: histórico e desenvolvimento. In: Fundamentos da circulação extracorpórea. vol. I. Rio de Janeiro:Centro Editorial Alfa Rio; 1995. p.3-33.
9. Braile DM, Godoy MF. História da cirurgia cardíaca. Arq Bras Cardiol. 1996;66(6):329-37.
10. Taylor KM. Cardiopulmonary bypass: principles and management. London:Chapman and Hall;1986. p.986.
11. Kolff WJ, Berk TJ. Artificial kidney: dialyzer with great area. Acta Med Scand. 1944;117:121-34.
12. Barrer RM, Skirrow G. Transport and equilibrium phenomena in gas-elastomer systems. I. Kinetic phenomena. J Polimer Sc. 1948;3:549-63.
13. Van Amerongen CJ. Influence of structures of elastomers on their permeability to gases. J Polymer Sc. 1950;5:307-32.
14. Kolff WJ, Effler DB, Groves LK, Peereboom G, Moraca PP. Disposable membrane oxygenator (heart-lung machine) and its use in experimental surgery. Cleve Clin Q. 1956;23(2):69-97.
15. Clowes Jr. GHA, Neville WE. Further development of a blood oxygenator dependent upon the diffusion of gases through plastic membranes. Trans Am Soc Artif Intern Organs. 1957;3:52-8.
16. Clowes Jr. GHA, Neville WE. The membrane oxygenator. In: Extracorporeal circulation. Springfield: Thomas;1958. p.81-100.
17. Peirce EC II, Rogers WK, Dabbs CH, Rawson FL. Clinical experience with the membrane lung used in conjunction with hypothermia. J Tn State Med Assoc. 1961;54(1):39-43.
18. Landé AJ, Edwards L, Bloch JH, Carlson RG, Subramanian V, Ascheim R et al. Prolonged cardiopulmonary support with a practical membrane oxygenator. Trans Am Soc Artif Intern Organs. 1970;16:352-6.
19. Kolobow T, Spragg RC, Pierce JE, Zapol WM. Extended term (to 16 days) partial extracorporeal blood gas exchange with the spiral membrane lung in unanesthetized lambs. Trans Am Soc Artif Intern Organs. 1971;17:350-4.
20. DATASUS, 2004.
21. Garcia AM. Circulação extracorpórea em crianças. Acesso em 11/09/2002. Disponível em: www.perfiline.com/artigos/artigos98/ceccrian.htm.
22. Souza MHL, Elias DO. Curso de perfusão neonatal. Acesso em 25/07/2005. Disponível em: www.perfiline.com
23. Gandolfi JF, Braile DM. Perspectivas de aplicação clínica da oxigenação extracorpórea por membrana (ECMO) sem auxílio circulatório em recém-nascidos. Rev Bras Cir Cardiovasc. 2003;18(4):359-63.
24. Moscardini AC, Godoy MF, Braile DM, Godoy JMP, Soares MJ, Brandi AC et al. Oxigenação extracorpórea por membrana e alterações hematológicas em estudo experimental. Rev Bras Hematol Hemoter. 2002;24(2):97-104.
25. Novello WP, Dias FT, Bergamasco N, Braile DM. Clinical evaluation of oximaster i membrane oxygenator. Proceeding of the European Medical & Biological Engineering Conference. 1999;37(2):66-7.
26. Novello WP, Braile DM. Avaliação da tendência ao vazamento de plasma em membranas utilizadas em circulação extracorpórea. In: Congresso Brasileiro de Engenharia Mecânica; 1999; Águas de Lindóia. Anais/CD Rom. Águas de Lindóia:Associação Brasileira de Ciências Mecânicas;1999.

 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license