INTRODUCTION
The effectiveness of coronary artery bypass grafting (CABG) was established by three great studies: European Coronary Surgery Study Group, Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group (VA) and Coronary Artery Surgery Study Group (CASS). These three studies established the superiority of the direct myocardial revascularization subgroups of patients with coronary arterial disease. The time-course follow-up of patients selected in these three studies showed that the benefits of surgical treatment compared with the benefits of clinical treatment decreased as time goes by. What was expected with CABG as a solution to treat coronary disease was frustrated by the longevity of saphenous bypass grafts.
It is known that the patency of saphenous vein grafts is inferior to arterial grafts. This is due, in part, to the fact that the normal saphenous vein wall has different structural and functional characteristics, which may be affected by high pressures mainly during vein preparation and its insertion in the arterial system. When implanted in the arterial circulation, a venous graft changes from a low-flow, non-pulsatile system at a low pressure to an ambient of pulsatile flow with high pressure and shear stress forces under significant hemodynamic changes. These hemodynamic alterations may be, at least in part, responsible for short- and long-term functional and morphological saphenous vein injuries that cause intima layer hyperproliferation followed by atheromatous changes that contribute to early graft thrombosis.
Remodeling dependence in the presence of intact endothelium and morphologic fluctuations reflect "shear stress" suggesting that the endothelium is a sensor of the local mechanical forces of fluids. The non-random distribution of atherosclerotic lesions observed in human patients and experimental projects, implicate the local hemodynamic forces as crucial factors in the development of this disease. Thus, hemodynamic forces are important stimuli of the vascular endothelium, playing a pivotal role in physiological and pathological processes (atherosclerosis, hypertension, thrombosis).
Many authors have studied the hemodynamic, functional and morphological alterations in saphenous veins when submitted to in vivo or in vitro arterial graft conditions. These studies follow several lines of research and frequently present with conflicting results. This review was motivated with the specific aim of analyzing published data and presenting some results of experiments carried out in our laboratory.
MECHANICAL FORCES THAT ACT ON THE VASCULAR INTIMA
Vascular endothelial cells are exposed to a variety of in vivo mechanical forces, specifically, shear stress due to blood flow, tensile stress from the compliance of the vessel wall and hydrostatic pressure from containment of blood within the vessel.
The effects of the hydrostatic pressure have only recently received attention, but results of several studies suggest that the responses of endothelial cells to hydrostatic pressure differ from those of shear and tensile stress.
These studies demonstrate that exposure of both bovine and human endothelial cells to sustained pressures, stimulates cell proliferation and alters cellular morphology, evidenced by cell elongation (without predominant cell alignment) concomitant to cytoskeletal reorganization [1-4]. Additionally, exposure of human endothelial cells to sustained pressures does not alter basal surface expression of intercellular adhesion molecules (ICAM-1), vascular cell adhesion molecules (VCAM-1) E-Selectin, CD31, or p96 [5]. These results are in contrast to the upregulation of ICAM-1 and the downregulation of VCAM-1 and E-Selectin [6] induced in endothelial cells by laminar "shear stress".
ENDOTHELIAL RESPONSES TO SHEAR STRESS IN VITRO
Evidence of the direct action of hemodynamic forces on the endothelial structure and its function were observed in in vitro studies in which human and animal endothelium cells were subjected to defined mechanical forces [7-9]. Results of these experiments imitate the in vivo response of the endothelium to different shear forces. Cells exposed to unidirectional "shear stress" elongated and became aligned in the direction of the laminar flow, changes that were accompanied by the formation of stress fibers and redistribution of actin fibers and microtubules. Under non-laminar forces, these cells become polygonal, without any specific alignment. The stress from laminar flow leads to exhaustion of the cellular cycle, while low levels of turbulent flow for periods of at least 3 hours activated the cellular cycle, but without evidence of cellular retraction or damage [10-12].
Response to "shear stress" begins immediately after the beginning of flow when sharp changes in the structure of the membrane, organization of the cytoskeleton and composition or even phosphorylation of adhesion proteins were observed [13-15]. This response continues with modifications in the activity and distribution of ionic channels across the endothelial cellular membrane [13,16], alterations in the intracellular calcium [16-18] as well as activation and phosphorylation of many signaling molecules including G proteins, MAP kinase and Erk [19,20]. Other immediate responses, such as the production of arachidonate metabolites and vasoactive mediators, are also detected [7,21-23]. Most of the immediate responses do not require protein synthesis and seem to involve regulation at the level of the enzymes or substratum availability.
Later responses (minutes to hours after the beginning of flow) include up- and down-regulation of endothelial molecules, many of which are key components of physiological or even pathological effector systems found in the vascular endothelium, that modulate thrombosis and hemostasis, vascular tonus, vascular growth and inflammatory reactions. What was verified is the fact that many of the molecules are regulated at the gene expression level, providing a useful paradigm for the investigation of gene regulation by biomechanical forces.
EFFECTS OF HYDROSTATIC PRESSURE ON ENDOTHELIAL CELL MORPHOLOGY
Cell morphology is a dynamic participant in, and indicator of, cellular function. Alterations in the cellular morphology are associated with cellular differentiation, proliferation, migration and cell-cell interactions [24]. In vivo vascular endothelial cells are elongated and aligned in the direction of the blood flow. However, in blood vessels, where flow is disturbed, these cells are rounded and present with 'cobblestone' morphology [25]. The cells of capillary vessels are flattened and single cells are organized around the vessel to form its lumen. In the angiogenesis process, endothelial cells undergo several morphological alterations in order to form new, small blood vessels; specifically, cells at the site of a new capillary elongate and then migrate, invading the extracellular matrix which originally surrounded the vessel wall [26]. Furthermore, initiation of either apoptosis or proliferation is regulated by the degree of cell extension and is independent of the total area of focal adhesion per cell. However, a synergistic effect exists between the total cell area and adhesive proteins on the cell surfaces, so that cells with surfaces with fibronectin and type I collagen are more susceptible to apoptosis than cells with vitronectin [27]. Thus, even though it is an important phenomenon related to its own origin, the morphologic alterations that happen when the cells are exposed to pressure may contribute to or even control changes in cellular function, such as an increment in cellular proliferation and changes in cellular protein expressions.
In vitro exposure of bovine and human endothelial cells to sustained pressures of 1.5-109 cm H2O for 1-9 days results in cell elongation without any predominant cellular alignment [1-4]. These changes in cell shape are accompanied by concomitant reorganization of the cell cytoskeleton. In particular, endothelial cells submitted to pressure lose their outlying characteristics of actin band and reorganize internal regions of the cytoskeleton to a web-like matrix of an aligned array of thick parallel stress fibers [1-4]. In addition, the cytoskeleton reorganizes from a single plane of cells maintained under controlled conditions to a multilayered structure after exposure to pressure. The number of layers (up to 5 distinct planes with 10 cm H2O) as well as the thickness of individual fibers depends on the magnitude and duration (1-7 days) of the applied pressure [3].
The changes in cell and cytoskeletal morphology that occur while endothelial cells are exposed to sustained hydrostatic pressure are similar to the changes observed in angiogenesis and during other cellular proliferation events. It is interesting to note that cell elongation and cytoskeleton alignment are similar to those of early angiogenesis. Furthermore, cell elongation (without predominant cell alignment) observed during exposure of endothelial cells to pressure is similar to the morphological changes observed during exposure to "shear stress" due to turbulent fluid. This mechanical force is also associated with increased cell proliferation and to morphological changes that result from controlled cell spreading that inhibits apoptosis and initiates endothelial cell proliferation in vitro [27]. Thus, the morphological changes that occur following exposure of endothelial cells to pressure suggest that these cells exhibit an active proliferative phenotype.
EFFECTS OF TRACTION ON THE HUMAN SAPHENOUS VEIN (HSV) ENDOTHELIUM
In in vitro experiments, HSV segments were stretched lengthwise demonstrating that this increases the metalloproteinases expression - enzymes that degrade the extracellular matrix - and the receptors of these enzymes, as well as stimulating cell proliferation, especially in the adventitia [28]. Growth factors and metalloproteinases act together favoring neointima formation. Metalloproteinases degrade the extracellular substance in which smooth muscle cells are soaked and release the support that maintains them in a latent state, at a low replication rate, thus facilitating their proliferation and migration. It was observed that increased metalloproteinases activity coincides with smooth muscle cell proliferation and neointima formation in sheep SV grafts implanted into the carotid artery circulation [29].
HSV is submitted to a certain manipulation during its harvesting and implant as grafts, such as its distention by high pressure saline solution to locate collateral branches and tie them and to select the segments that will be used as grafts. In HSV distended experimentally at the same pressure that is used in surgeries prior to selecting the segment to be implanted (350 mmHg for 2 minutes), an increase of the RNA messenger expression has been described for genes that induce cell growth factors [30]. The inhibition of the expression of these genes in segments of sheep SV, before implanting as grafts in the carotid artery, reduces neointima formation in the first months after the procedure [31].
A recent investigation, using segments of HSV during minimally invasive harvesting, evidenced impaired endothelial function. It was concluded that the possible cause of endothelial dysfunction could be due to traction lesions [32].
ENDOTHELIAL GENE REGULATION CAUSED BY SHEAR STRESS
Remodeling of blood vessels accompanies physiological and pathological processes such as angiogenesis and vasculogenesis, atherosclerosis, hypertension and restenosis. Vessel remodeling occurs in response to both biochemical and biomechanical stimuli, and it has been demonstrated to be independent of the presence of an intact endothelial layer. Because of their anatomical position, endothelial cells are constantly exposed to hemodynamic forces generated by the blood flow, forces that consist of the stress of fluid, shear stress, cyclic strain and pressure. These forces affect structure and function of endothelial cells, changes that are often mediated by the induction or inhibition of endothelial genes.
Recently some endothelial genes have been identified as regulators of these hemodynamic forces. These "shear stress" response elements (SSREs) bind to transcription factors, including nuclear factors kappa B (NFkapaB) and ATc2 (NFATc2), and the Sp1, Fos and Jun transcription factors, which are activated by hemodynamic forces.
EFFECTS OF HYDROSTATIC PRESSURE ON THE GENE EXPRESSION OF THE ENDOTHELIAL CELL SURFACE
In addition to the morphologic and cytoskeletal changes, exposure of endothelial cells to sustained pressure may alter the basal or stimulated expression of cell surface antigens such as á5á1 and E-selectin (CD62E), a cytokine-induced adhesion molecule which is thought to play a role in leukocyte trafficking and in angiogenesis. Integrin 51 plays a role in cell-cell and cell-matrix interactions as well as in the transduction of mechanical stimuli in endothelial cells. Exposure of bovine aortic endothelial cells to 52 cm H2O pressure for periods of 12-48 hours results in increased clustering of vinculin, talin and integrin 5 in focal adhesion plaques, in laminin and collagen type IV fibril thickening in the extracellular matrix, and in increased deposition of the fibronectin and laminin (but not vitronectin) in the extracellular matrix [33]. Exposure of human umbilical vein endothelial cells to 4 cm H2O sustained pressure for one day does not affect the basal cell surface expression of intercellular adhesion molecule-1 (ICAM-1 or CD54), vascular cell adhesion molecule1 (VCAM-1 or CD106), E-Selectin (CD62E) or CD31 and platelet-endothelial cell adhesion molecule (p96) [5]. However, exposure of human umbilical vein endothelial cells to pressure does appear to either prolong or enhance the E-Selectin expression that occurs following contact of these cells to 100 ng/mL of E. coli lipopolysaccharide [4].
EFFECTS OF HYDROSTATIC PRESSURE ON THE PROLIFERATION OF ENDOTHELIAL CELLS
Most endothelial cells in vivo exhibit a very low turnover rate (<1 cell division/year). Endothelial cell proliferation, however, is observed under a number of normal physiological conditions including wound healing, embryogenesis and ovulation. In addition, a number of pathological states, including glaucoma-induced retinal microangiopathy, rheumatoid arthritis and solid tumor growth are associated with increased endothelial cell proliferation. Interestingly, these pathologies (as well as wound healing) are also associated to localized elevations of hydrostatic pressure.
The in vivo association between increased hydrostatic pressures and increased endothelial cell proliferation may be only incidental, although a causal relationship cannot be excluded. A possible link is suggested by in vitro studies from several laboratories which have demonstrated that exposure of bovine, porcine and human endothelial cells to sustained hydrostatic pressures in the range of 1.5 - 260 cm H2O for periods of 1 to 9 days stimulates endothelial cell proliferation and loss of contact-inhibited growth, leading to multiple-layering.
DISCUSSION
The great saphenous vein continues to be the most common graft together with the pediculated internal thoracic artery for CABG, even after considering the advantages of arterial grafts that are adapted to pressure and present with better long-term results. This adaptation depends on the endothelium paracrine function that, on releasing vasodilator and anti-platelet substances prevents spasms, thrombosis, and proliferation and, even protects against atherosclerotic mechanisms.
Many studies have investigated the reasons for the functional inferiority of the saphenous vein, attributing great importance to possible traumatic and/or functional lesions, during its harvesting and preparation for revascularization. Once harvested, the vein is cannulated, gradually pressurized to locate and tie collateral branches previously not identified. Patency rates may be associated to vein lesions during its harvesting and preparation [34-37]. Although there are proven functional advantages of minimal manipulation techniques of vein grafts ("non-touch" techniques), cannulation and vein pressurization, due to its practicality, is still an almost universal practice.
In the 1960s and 1970s morphologic injuries, not only of the endothelium but also of the other vein wall layers, were clearly established. In these studies vein pressurization would be a prohibitive practice that was not observed in the evolution of CABG. Hence, a great challenge was to discover to what point the morphologic lesions caused by pressurization were associated to functional lesions. In other words, does a "functional barotrauma" exist in these pressurized veins?
In the 1990s, working in the Mayo Clinic (Rochester, MN), we verified that in vivo infusions of cardioplegic solution (above 600 mmHg), did not induce endothelial dysfunction (in vitro study using "organ chambers") based on a non-significant decrease in nitric oxide release in normal canine coronary arteries [37].
Another investigation of our laboratory, which is maybe the one with the best design protocol, was carried out to study the relationship between the degree of vein distention pressure and the level of structural, biochemical and functional lesions of human saphenous vein vascular wall. In this study, segments of human saphenous vein were inflated using pressures of from 100 to 300 mmHg before in vitro vascular reactivity studies in organ baths. Apart from functional studies, structural lesions were evaluated by high resolution microscopy and by NOS immunohistochemistry expression.
Segments of saphenous vein distended using a pressure of 100 mmHg maintained their functional capacity to potassium chloride (90 mmol/L) and phenylephrine (10-6 mol/L), but those pressurized to 300 mmHg lost their reactivity to the same pharmacologic agents. Veins distended with 300 mmHg also had endothelium-dependent relaxation impairment to acetylcholine (10-10 to 10-6 mol/L) and bradykinin (10-10 to 10-6 mol/L). Quantitative studies of structural endothelial injury were apparent when a pressure of 300 mmHg was used, but endothelial cells maintained strong immunohistochemistry staining for endothelial syntheses. Unlike veins submitted to 100 mmHg, veins pressurized to 300 mmHg showed great areas of nude endothelium. This study concluded that distention of saphenous veins at pressures equivalent to high arterial blood pressures in humans, results in structural and biochemical alterations of the endothelium that are not associated to immediate functional alterations. In other words, the endothelium presents a great functional reserve.
During an investigation of HSV, data were collected on the effect of mechanical forces on endothelial function. The arterial circulation consists of muscular and elastic vessels with large diameters as well as arterioles and pre-capillary vessels, constantly exposed to hemodynamic forces that vary greatly in size, frequency and direction. These forces consist of pressures acting on the vascular wall, creating a cyclical effort, and "shear stress" acting along the vessels. This "shear stress" creates a shear force on the endothelium surface.
When saphenous veins are inserted in the arterial circulation they suffer the action of these physical phenomena. Additionally, traction forces affect the functionality of the veins. But, the design of our ongoing laboratory investigations were just centered in the hydrostatic pressure generated by the infusion of physiological Krebs solution as occurs during the harvesting and preparation of the saphenous veins. The studied vein segments all originated from the saphenous vein close to the internal right leg malleolus. These veins were manipulated little; they did not present with varicosity or macroscopic inflammatory reactions. They were not initially pressurized nor did they come in contact with any vasodilator drugs. We studied twenty veins from different patients, a situation subject to criticism, but we believe this is a reasonable number "to dilute" the extreme variability of vessels from patient to patient. There is one possible bias, which is the use of the same initial vein segment with the possibility of variations in endothelial function in different regions of the harvested vein. This possible bias was removed by a recent Texas Heart Institute publication which stated that, in vitro saphenous vein studies using acetylcholine and sodium nitroprusside did not reveal functional differences between saphenous vein segments of the thigh and the initial segment of the leg [38].
Our main results were 1) from a 200 mmHg pressurization, a tendency to decrease CD34 expression was observed which became statistically significant at 300 mmHg (Figure 1); 2) high resolution microscopy did not reveal differences in the endothelium intimal surface in respect to the percentage cover, perimeter or external and internal diameters.; 3) a non-significant increase in eNOS expression was seen at 100 mmHg with a tendency to decrease at 200 mmHg becoming significant at 300 mmHg; 4) the eNOS expression in the smooth cell muscle was equivalent to the endothelial expression and did not suffer any effect with pressurization (Figure 2); 5) there was no significant nNOS expression in either the endothelium or smooth muscle; 6) there was no significant iNOS expression in the endothelium of the vein unlike in the smooth muscle that showed the expression of this enzyme to be unaffected by the experimental pressurizations (Figure 3); 7) the nitrotyrosine expression was insignificant; 8) there was a decrease of MDA in the veins submitted to pressures of 300 mmHg; 9) there were no changes in nitrite/nitrate in the vein tissue as measured by chemiluminescence, and; 10) the in vitro vascular reactivity (contractions and relaxation) was not significantly affected by pressure.
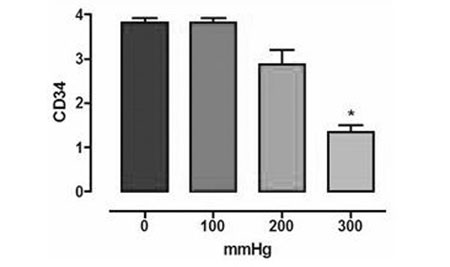
Fig. 1 - Immunohistochemical expression of CD 34 (n=20, ANOVA, p < 0,05). Asterisk indicates statistically significant difference
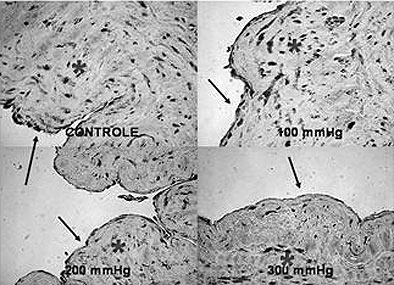
Fig. 2 - Immunohistochemical for eNOS. The arrows indicate the almost the eNOS expression in the endothelium of the saphenous vein and the asterisks indicate regions of eNOS expression in the vascular smooth muscle (400x)
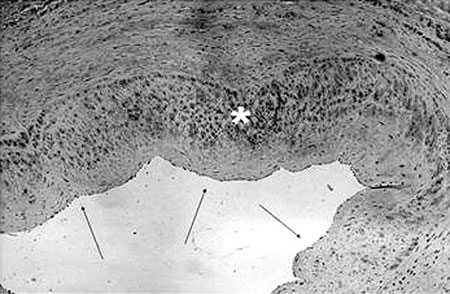
Fig. 3 - Immunohistochemical of iNOS. The arrows indicate the almost null expression of iNOS in the endothelium of the saphenous vein and the asterisk indicates an exuberant expression of iNOS in the vascular smooth muscle (100x)
In this research line an excellent experimental paper was published by the group of the Heart Institute of the Hospital of Clinics - FMUSP (INCOR). Forty sections of SV were cultivated "ex-vivo" under venous hemodynamic conditions (VHC) (without pressure; flow: 5 mL/min) and under arterial hemodynamic conditions (AHC) (pressure: 80 mmHg; flow: 50 mL/min). The following variables were analyzed: cellular viability (MTT assay) cellular density (Hoeschst 33258 staining) and apoptosis (TUNEL assay), before the procedure and 1, 2 and 4 days after. Analysis of the SV sections by "cDNA microarray" determined precociously changed molecular targets in the veins cultivated under arterial conditions.
The identification of these targets was achieved using a RNA homogenized pool of vein sections, interacting on slides with 16,000 pre-determined human genes (Agilent Technologies slide). Genes with changed expressions were verified by real time PCR in the veins of 16 patients. There was a gradual reduction in the cellular density and in the tissue viability of saphenous veins cultivated under AHC, whereas no alterations were observed in saphenous veins cultivated under VHC for up to 4 days. In the AHC Group there were signs of a cellular apoptotic process (positive - TUNEL) from the first day after cultivation. In the VHC group these alterations were not observed. Although the cellular density was the same in veins submitted to arterial conditions after 24 hours of cultivation, many cells already showed signs of the apoptotic process. The Oncogene 3 and the Interleukin 1ß were the most common sites with alterations identified in this research. The Oncogene 3 expression was elevated in 11 (68.7%) of the veins cultivated under AHC, and the Interleukin 1ß expression was elevated in 9 (56.2%) vein sections (p<0.05). The "ex vivo" study model was able to mimic events that occur "in vivo" with SVs utilized in coronary artery bypass grafting. In the AHC Group early loss of cellular viability (apoptosis) and significant elevation in the Oncogene 3 and Interleukin 1ß genic expressions were observed. The long-term follow up of these patients is important to determine the real effect of these immediate changes in the patency of vein grafts [39].
By comparing human saphenous veins obtained by conventional techniques and "non-touch" techniques, the literature shows a reduction of the eNOS expression and NO release impairment in veins that were handled more during extraction/dissection [40,41]. The immunohistochemistry data of our investigations confirm this eNOS expression impairment for the most manipulated saphenous veins. A comparison between minimally handled saphenous veins with veins removed by the conventional technique would be interesting, keeping the pressurization at levels under 100 to 150 mmHg.
In our investigations only immunohistochemistry data was able to conclude excessive saphenous vein distension results, without any doubt, in endothelial function impairment. Our experimental observations revealed that pressurization of up to 300 mmHg does not affect the saphenous vein from the pharmacologic point of view, and the in vitro vascular reactivity. It causes endothelial dysfunction (CD34), does not generate free radicals by lipid peroxidation (normal MDA and negative expression of nitrotyrosine), it does not affect the nitrite/nitrate tissue levels and it can act as inflammatory stimuli (iNOS expression) in the vascular smooth muscle. That allows speculation that, over a short period injury does not occur to saphenous veins removed and pressurized with great caution. But, surgical manipulation somehow creates a type of "molecular scar" that can affect, over varied periods of time, the coronary vein graft patency.
Our investigations, as already mentioned, are maybe the most complete ever performed, confirming the subjective impression that the factors that cause coronary artery bypass occlusion play an arduous endothelial duel; "dynamiting our bridges". At the beginning of the vein segment transposition to the arterial territory of great resistance with the functional specialization of its wall, is an "announced death" within an unpredictable time.
To conclude this review, it is mandatory to mention a fundamental book that treats, specifically, the mechanical force's action on the endothelium. It is a book edited by Lelkes in 1999, which has been a kind of "Bible" for the investigations in our laboratory, and it was the basic text used for the current review [42].
REFERENCES
1. Acevedo AD, Bowser SS, Gerritsen ME, Bizios R. Morphological and proliferative responses of endothelial cells to hydrostatic pressure: role of fibroblast growth factor. J Cell Physiol.1993;157(3):603-14.
2. Sumpio BE, Banes AJ, Levin LG, Johnson G Jr. Mechanical stress stimulates aortic endothelial cells to proliferate. J Vasc Surg. 1987;6(3):252-6.
3. Salwen S. MS Thesis. New York:Department of Biomedical Engineering, Rensselaer Polytechnic Institute;1994.
4. Schwartz E. Mechanotransduction of sustained hydrostatic pressure by human endothelial cells [PhD Thesis]. New York:Department of Biomedical Engineering, Rensselae lnstitute;1999.
5. Schwartz E, Bizios R, Gerritsen M. Effects of sustained hydrostatic prf expression of endothelial cell-Ieukocyte adhesion molecules. In: Reeves J, ed. Pulmonary edema. New York:Futura Publishing Company;1998. p.195-203.
6. Sampath R, Kukielka GL, Smith CW, Eskin SG, Mclntire LV. Shear stress-mediated changes in the expression of leukocyte adhesion receptors on human umbilical vein endothelial cells in vitro. Ann Biomed Eng. 1995;23(3):247-56.
7. Frangos JA. Physical forces and the mammalian cell. San-Diego:Academic Press;1993.
8. Resnick N, Gimbrone MA Jr. Hemodynamic forces are complex regulators of endothelial gene expression. FASEB J. 1995;9(10):874-82.
9. Panaro NJ, McIntire LV. Flow and shear stress effects on endothelial cell function. In: Sumpio BE, ed. Hemodynamic forces and vascular cell biology. Austin:R.G. Landes Company;1993. p.5.
10. Dewey CF Jr, Bussolari SR, Gimbrone MA Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1988;103(3):177-85.
11. Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75(3):519-60.
12. Nerem RM, Harrison DG, Taylor WR, Alexander RW. Hemodynamics and vascular endothelial biology. J Cardiovasc Pharmacol. 1993;21(Suppl 1):S6-10.
13. Davies PF. Overview: temporal and spatial relationships in shear stress-mediated endothelial signalling. J Vasc Res. 1997;34(3):208-11.
14. Davies PF. Mechanisms involved in endothelial responses to hemodynamic forces. Atherosclerosis. 1997;131(suppl):S15-7.
15. Isales C, Rosales O, Sumpio BE. Mediators and mechanisms of cyclic strain and shear s-induced vascular responses. In: Sumpio BE, ed. Hemodynamic forces and vascular cell biology. 1993.
16. Olesen RG, Clapham DE, Davies PF. Hemodynamic shear stress activates a K+ current in vascular endothelial cells. Nature. 1988;331(6152):168-70.
17. Dull RO, Davies PF. Flow modulation of agonist (ATP) response (Ca2+) coupling in vascular endothelial cells. Am J Physiol. 1991;261(1 pt 2):H149-54.
18. Shen J, Luscinkas FW, Connolly A, Dewey CF Jr, Gimbrone MA Jr. Fluid shear stress modulates cytosolic free calcium in vascular endothelial cells. Am J Physiol. 1992;262(2 Pt 1):C384-90.
19. Takahashi M, Ishida T, Traub O, Corson MA, Berk BC. Mechanotransduction in endothelial cells: temporal signaling events in response to shear stress. J Vasc Res.1997;34(3):212-9.
20. Ishida T, Takahashi M, Corson MA, Berk BC. Fluid shear stress mediated signal transduction: how do endothelial cells transduce mechanical force into biological responses? Ann NY Acad Sci. 1997, 811:12-24.
21. Furchgott RF. Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep. 1999;19(4):235-51.
22. Rubanyi GM, Romero JC, Vanhoutte PM. Flow induced release of endothelium-derived relaxing factor. Am J Physiol. 1986;250(6 Pt 2):HI145-9.
23. Yoshizumi M, Kurihara H, Sugiyama T, Takaku F, Yanagisawa M, Masaki T, et al. Hemodynamic shear stress stimulates endothelin production by cultured endothelial cells. Biochem Biophys Res Commun. 1989;161(2):859-64.
24. Sims JR, Karp S, lngber DE. Altering the cellular mechanical force balance results in integrated changes in cell, cytoskeleta, and nuclear shape. J Cell Sci 1992;103(Pt 4):1215-12.
25. Thubrikar MJ, Robicsek F. Pressure-induced arterial wall stress and atherosclerosis. Ann Thorac Surg. 1995;59(6):1594-603.
26. Stromblad S, Cheresh DA. Cell adhesion and angiogenesis. Trends Cell Biol. 1996;6(12):462-8.
27. Chen CS, Marksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276(5317):1425-8.
28. Meng X, Mavromatis K, Galis ZS. Mechanical stretching of human saphenous vein grafts induces expression and activation of matrix-degrading enzymes associated with vascular tissue injury and repair. Exp Mol Pathol. 1999;66(3):227-37.
29. Southgate KM, Mehta D, Izzat MB, Newby AC, Angelini GD. Increased secretion of basement-membrane degrading metalloproteinases in pig saphenous vein into carotid artery interposition grafts. Arterioscler Thromb Vasc Biol. 1999;19(7):1640-9.
30. Galea J, Armstrong J, Francis SE, Cooper G, Crossman DC, Holt CM. Alterations in c-fos expression, cell proliferation and apoptosis in pressure distended human saphenous vein. Cardiovasc Res. 1999;44(2):436-48.
31. Mannion JD, Ormont ML, Magno MG, O'Brien JE, Shi Y, Zalewski A. Sustained reduction of neointima with c-myc antisense oligonucleotides in saphenous vein grafts. Ann Thorac Surg. 1998;66(6):1948-52.
32. Cook RC, Crowley CM, Hayden R, Gao M, Fedoruk L, Lichtenstein SV, et al. Traction injury during minimally invasive harvesting of the saphenous vein is associated with impaired endothelial function. J Thorac Cardiovasc Surg. 2004;127(1):65-71.
33. Thoumine O, Nerem RM, Girard PR. Oscillatory shear stress and hydrostatic pressure modulate cell-matrix attachment proteins in cultured endothelial cells. In Vitro Cell Dev Biol Anim. 1995;31(1):45-54.
34. Soyombo AA, Angelini GD, Bryan AJ, Newby AC. Surgical preparation induces injury and promotes smooth muscle cell proliferation in a culture of human saphenous vein. Cardiovasc Res. 1993;27(11):1961-7.
35. Bonchek LI. Prevention of endothelial damage during preparation of saphenous vein for bypass grafting. J Thorac Cardiovasc Surg. 1980;79(6):911-5.
36. Angelini GD, Breckenridge IM, Williams HM, Newby AC. A surgical preparative technique for coronary bypass grafts of human saphenous vein which preserves medial and endothelial functional integrity. J Thorac Cardiovasc Surg. 1987;94(3):393-8.
37. Evora PR, Pearson PJ, Schaff HV. Impaired endothelium-dependent relaxation after coronary reperfusion injury: evidence for G-protein dysfunction. Ann Thorac Surg. 1994;57(6):1550-56.
38. Golbasi I, Tasatargil A, Aksoy NH, Sadan G, Karasu E, Turkay C, et al. A functional and histopathological comparison of proximal and distal saphenous vein contractility and morphology. Tex Heart Inst J. 2005; 32(3):287-93.
39. Dallan LAO, Miyakawa AA, Lisboa LA, Abreu Filho CA, Campos L, Borin T, et al. precocious structural and molecular (cDNA) changes in the human saphenous veins cultivated under arterial hemodynamic conditions. Braz J Cardiovasc Surg. 2004;19(2):126-35.
40. Tsui JC, Souza DS, Filbey D, Karlsson MG, Dashwood MR. Localization of nitric oxide synthase in saphenous vein grafts harvested with a novel "no-touch" technique: potential role of nitric oxide contribution to improved early graft patency rates. J Vasc Surg. 2002;35(2):356-62.
41. Souza DSR, Dashwood MR, Tonazi A, Johansson B, Buffolo E, Lima R, et al. preparation of the saphenous vein for coronary artery bypass grafting: a new technique "no touch" that maintains the vein wall integral and provides high immediate patency. Rev Bras Cir Cardiovasc. 2003;18(4):303-11.
42. Lelkes P, Gimbrone MA Jr. Mechanical forces and the endothelium. Amsterdam:Harwood Academic Publishers;1999.



 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license