INTRODUCTION
Conventional treatment of descending aorta dissections, Stanford type-B aortic dissection, includes both clinical treatment and surgical approach [1-3]. Initial treatment for uncomplicated acute dissections is medical; however, when it is associated to aching pain complications, sealed rupture, and ischemia of organs and limbs, the treatment is surgical [4].
Nevertheless, these two therapeutic options have limitations. Medical treatment for uncomplicated cases presents variable in-hospital mortality about 10%; in a 4-year follow-up 20% to 30% of the patients can develop significant dilations and 18% wi8ll develop rupture [5,6]. Conventional surgical treatment is followed by high morbidity and mortality, especially when it is associated to hemodynamic instability secondary to rupture, mesenteric ischemia, or chronic obstructive pulmonary disease [7].
Another surgical treatment option for such patients is the endovascular approach [8]. This therapeutic approach has evolved since its beginnings, especially on account of prosthesis development and improvement of its usage in clinical practice. Currently, some centers suggest that uncomplicated acute Stanford type-B aortic dissections when submitted to endovascular management present a better outcome when in comparison to the natural course of the disease as in intra-hospital phase as in medium-term follow-up [2,3,9,10]. In acute phase complications, it seems there is no doubt concerning the benefit of endovascular treatment over the conventional one [11,12].
In chronic Stanford type-B aortic dissections, the endovascular approach excitingly initiated presented complications especially related to flow persistence in the false lumen secondary to the existing reentries in the distal portions of thoracic aorta, in the abdominal portion of near to the abdominal vessels, or even the existing complications in iliac arteries, which would maintain the false lumen pressurized. This would prevent that the treatment with occlusion of the main entry site in the initial portion of the descending aorta could lead to the thrombosis of the false lumen of the delaminated aorta to its depressurization which would result in the decrease of the vessel [10,13-15].
With the aim of evaluating endovascular treatment efficacy in chronic descending aortic dissections, taking into consideration abdominal and thoracic aorta diameters, and the presence of flow into the false lumen of the aorta in both segments in the course of time, we undertook this research.
METHODS
Between April 2003 to February 2006, 11 patients with chronic Stanford type-B aortic dissection underwent endovascular treatment of descending aorta through femoral artery approach were enrolled in this study. Their clinical characteristics are listed in Table 1.
The study protocol was approved by the Research and Ethics Committee of Heart Institute (InCor), Faculty of Medicine, University of São Paulo as a research protocol SDC 2658/05/078. Regarding treatment and necessity of periodical reevaluations, a free written informed consent was obtained from the patients.
An angiotomographic scan was performed to evaluate and diagnose the anatomic characteristics of these patients' aortas.
The inclusion criteria were patients with chronic Stanford type-B aortic dissection with main entry site in the proximal portion of the descending aorta; diameters of the thoracic and abdominal segments of the aorta e" 5,5 cm and d" 4,5 cm, respectively; and access route and landing zone with favorable anatomic characteristics allowing free movement and fixation of the endoprosthesis. Exclusion criteria were the impossibility of patient's late follow-up; celiac trunk, superior mesenteric artery, or the two renal arteries originating from the vessel false lumen; and thrombosed aorta false lumen postoperatively.
The study was carried out in a consecutive and prospective fashion, in which all the patients who presented with the abovementioned criteria and agreed to participate in the study were included. Neither of the patients refused to participate. Over the same period, 18 patients with chronic Stanford type-B aortic dissection underwent conventional surgical repair due to significant dilation of the entire aorta and not only of the thoracic segment.
Operation performed
Patients under general anesthesia underwent aortography through right axillary artery or brachial artery approach. An adequacy of the prosthesis to be used was defined prior to the procedure by means of the measurements performed in the angiotomographic study. Endoprostheses were deployed in the right femoral artery as previously described [16]. One patient underwent a combined procedure of angioplasty with right coronary artery stent graft before the aortic procedure.
Endoprosthesis were used in an average of 1.3 endoprostheses per patient (seven patients received one endoprosthesis and the remaining four patients two endoprostheses). Self-expandable endoprosthesis, Braile Biomédica®, (Braile Biomédica, São José do Rio Preto, Brasil) and Apolo (Nano Endoluminal S/A, Florianópolis, Brasil) were used.
The sizes used in the majority of the patients were 34 mm X 90 mm and 34 mm X 150 mm.
Patients' follow-up
All patients underwent control angiotomography within six months, one year, and yearly thereafter to evaluate remodeling of the thoracic and abdominal aortas after endoprosthesis placement. The size of descending and abdominal aorta diameters were observed in course of time, thus in the presence of thrombosis or blood flow in the false lumen of the thoracic and abdominal segments of the aorta. Therefore, for purposes of study, on radiography both descending and abdominal aortas were divided into two segments each as follows: segment 1 was defined as that one extending from the left subclavian artery up to the projection in the aorta of the first dissection of the pulmonary artery trunk. The second segment was defined as that one extending from the first image after the segment 1 up to the portion of the aorta crossing the diaphragm. Segment 3 corresponds to the abdominal portion of the aorta extending from the diaphragm up to the more distal renal artery, and the segment 4 is that one extending from the first image after the segment 3 up to the aortic bifurcation in the iliac arteries.
Statistical analysis
Continuous variables were expressed as mean ± SD and categorical variables were expressed using percentages. Comparisons between different surgical data (before vs after), and during follow-up were done with two-way analysis of variance (ANOVA) with repeated measures and Chi-square test, respectively. Analyses were performed with SPSS (version 13; SPSS, Chicago, IL).
RESULTS
No patient presented endoleak type I immediately after the procedure. One patient during second endoprosthesis placement presented proximal displacement of the first endoprosthesis with subocclusion of left carotid artery. The patient underwent an emergency crossover carotid-carotid grafting. Another patient underwent the combined procedure with angioplasty and stent placement in the coronary artery. There was no in-hospital mortality.
During follow-up period, length ranging from 9 to 43 months (mean of 28 months), one patient has stroke 14 months after the procedure; two patients presented recurrent thoracic pain (one of them due to coronary intrastent lesion, that one performed simultaneously to dissection of the aorta, and the other due to increase of dissected aorta diameter). The remaining of the patients (72.7%) is asymptomatic for thoracic pain. One patient underwent thoracoabdominal aorta replacement with iliac-femoral extension to the left 15 months after the initial procedure. Other two patients are waiting conventional repair due to increased aortic diameter during follow-up, despite the stent placement (27.3%). There was no late mortality.
Table 2 shows the presence or not of blood flow in the false lumen in the four aortic segments describe above after the aortic endoprosthesis placement for occlusion of the main entry dissection site. During the follow-up period, initial behavior changing was not observed, i.e., the thrombosis occurred in almost 73% of the patients in segment 1 neither spread to other aortic segments nor raised the number of patients with thrombosis in this portion of the aorta, neither did in the others, as shown in subsequent angiotomographic scans.
Figure 1 shows the thoracic aorta diameters of all patients screened in the course of time. Figure 2 shows the abdominal aorta diameters of all patients screened over the same period. Figure 3 shows surgical treatment failure with stent in changing the outcome of the patients with chronic dissection when considering the reduced thoracic and abdominal segments of the aorta in these patients. Despite endoprosthesis interrupting the perfusion flow of the thoracic and abdominal aortic false lumen in 73% and 18% of the patients, respectively, this did not influence the reductions in abdominal and thoracic aorta diameters during the follow-up period.
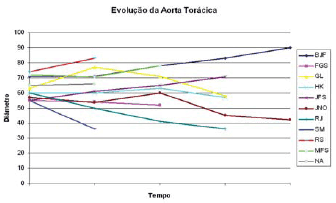
Fig. 1 - Development of the largest thoracic aortic diameters in the course of time
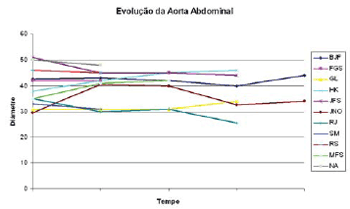
Fig. 2 - Development of the largest abdominal aortic diameters in the course of time
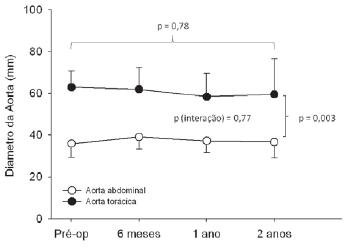
Fig. 3 - Mean variation of the thoracic and abdominal aortic diameters in the course of time, where it is observed the absence of variation of the diameters after endovascular treatment
Despite controversies regarding the optimal treatment in the initial approach of Stanford type-B dissections, the clinical treatment for uncomplicated patients still remains [10,17], although some authors have already identified that the false lumen with flow and a dissected aorta diameter larger than 40 mm are related to increase complications during the follow-up period of these patients [18,19].
Other authors, however, have shown that the patients with type-B dissections undergoing endovascular treatment in the acute phase presented more favorable outcomes than those observed with clinical treatment [2,20]. Randomized prospective studies aiming at comparing immediate and late outcomes of uncomplicated patients clinically treated with those undergoing endovascular treatments should be further undertaken [21].
When endovascular treatment of acute and chronic phases of type-B dissection are compared, thrombosis of the false lumen is found to be more frequent when the stent is placed on the acute phase due to the higher number of reentries of the chronic cases [5].
The aim of endovascular treatment in aortic dissection is to interrupt the false lumen flow with consequent depressurization of the aorta and reduction of the overall aortic diameter. The reduction of the diameter of the dilated aorta maintains the patient at risk of death and questions the efficacy of this therapeutic method for this specific purpose.
The efficacy of the false lumen thrombosis followed by the reduction of the thoracic aorta diameter in chronic Type-B dissections after stent graft treatment was described; however, it has occurred in less than 50% of the patients in the intra-hospital period and in less than one third of the times during late follow-up [6]. In the abdominal portion of the aorta, therapeutic failure is still higher, where neither most of the times one can observe false lumen thrombosis nor reduction of its diameters [7].
Bockler et al. [22] reported that associated to the endovascular treatment low capacity to lead to complete false lumen thrombosis of the delaminated aortas, the necessity of early reintervention is high being 19%, 27%, and 32% in 1, 2, and 5-year postoperative follow-up, respectively. Eggebrecht et al. [21] in a meta-analysis study also found an occurrence of high reintervention, 12% in less than a 2-year period.
Similarly, to our results the Swedish multicenter study presented the false lumen thrombosed along the stent graft fixation in 80% of the patients. However, in the distal portion of the thoracic aorta without stent grafting, false lumen flow remained persistent in 50% of the patients. It was observed that only 5% of the patients presented with aortic enlargement of the stent grafted area [23]. Contrary to the abovementioned, Won et al. [24] reported a significant decrease in diameter of the thoracic false lumen in a 100% of the patients. However, in all patients with aortic dissection, the abdominal aorta was not significantly changed in size and shape and their false lumen flows remained persistent.
Akutsu et al. [25] observed that patients with type-B dissections and false lumen flow in comparison to those with the same diagnosis and thrombosed false lumen, the patency of the false lumen was an independent risk factor for dissection-related death and complications. What makes us thing that if the stents do not lead to complete thrombosis of the false lumen of the delaminated aorta and to the consequent decrease of aortic diameters, is it justifiable to use it in the chronic Stanford type-B dissections?
Despite the small number of patients enrolled in this study, our results are in accordance with nearly all the studies found in the literature. This study also showed that to the patients undergoing chronic type-B Stanford dissection, the endovascular stent graft aortic treatment is not effective, once it does not decrease the aorta diameters.
REFERENCES
1. Albuquerque LC, Braile DM, Palma JH, Saadi EK, Gomes WJ, Buffolo E. Diretrizes para o tratamento cirúrgico das doenças da aorta da Sociedade Brasileira de Cirurgia Cardiovascular. Rev Bras Cir Cardiovasc. 2007;22(2):137-59. [
MedLine]
2. Xu SD, Huang FJ, Yang JF, Li ZZ, Wang XY, Zhang ZG, et al. Endovascular repair of acute type B aortic dissection: early and mid-term results. J Vasc Surg. 2006;43(6):1090-5. [
MedLine]
3. Shimono T, Kato N, Yasuda F, Suzuki T, Yuasa U, Onoda K, et al. Transluminal stent-graft placements for the treatments of acute onset and chronic aortic dissections. Circulation. 2002;106(12 Suppl 1):I241-7. [
MedLine]
4. Miller DC. The continuing dilemma concerning medical versus surgical management of patients with acute type B dissections. Semin Thorac Cardiovasc Surg. 1993;5(1):33-46. [
MedLine]
5. Juvonen T, Ergin MA, Galla JD, Lansman SL, McCullough JN, Nguyen K, et al. Risk factors for rupture of chronic type B dissections. J Thorac Cardiovasc Surg. 1999;117(4):776-86. [
MedLine]
6. Genoni M, Paul M, Jenni R, Graves K, Seifert B, Turina M. Chronic beta-blocker therapy improves outcome and reduces treatment costs in chronic type B aortic dissection. Eur J Cardiothorac Surg. 2001;19(5):606-10. [
MedLine]
7. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International Registry of Acute Aortic Dissection (IRAD): new insight into an old disease. JAMA. 2000;283(7):897-903. [
MedLine]
8. Buffolo E, da Fonseca JH, de Souza JA, Alves CM. Revolutionary treatment of aneurysms and dissections of descending aorta: the endovascular approach. Ann Thorac Surg. 2002;74(5):S1815-7.
9. Bortone AS, De Cillis E, D´Agostino D, Schinosa LLT. Endovascular treatment of thoracic aortic disease: four years of experience. Circulation. 2004;110(11 Suppl 1):II262-7. [
MedLine]
10. Lee KH, Won JY, Lee DY, Choi D, Shim WH, Chang BC. Elective stent-graft treatment of aortic dissections. J Endovasc Ther. 2004;11(6):667-75. [
MedLine]
11. Nienaber CA, Fattori R, Lund G, Dieckmann C, Wolf W, von Kodolitsch Y, et al. Nonsurgical reconstruction of thoracic aortic dissection by stent-graft placement. N Engl J Med. 1999;340(20):1539-45. [
MedLine]
12. Dake MD, Kato N, Mitchell RS, Semba CP, Razavi MK, Shimono T, et al. Endovascular stent-graft placement for the treatment of acute aortic dissection. N Engl J Med. 1999;340(20):1546-52. [
MedLine]
13. Gaxotte V, Thony F, Rousseau H, Lions C, Otal P, Willoteaux S, et al. Midterm results of aortic diameter outcomes after thoracic stent-graft implantation for aortic dissection: a multicenter study. J Endovasc Ther. 2006;13(2):127-38. [
MedLine]
14. Won JY, Suh SH, Ko HK, Lee KH, Shim WH, Chang BC, et al. Problems encountered during and after stent-graft treatment of aortic dissection. J Vasc Interv Radiol. 2006;17(2 Pt 1):271-81. [
MedLine]
15. Chen S, Yei F, Zhou L, Luo J, Zhang J, Shan S, et al. Endovascular stent-grafts treatment in acute aortic dissection (type B): clinical outcomes during early, late, or chronic phases. Catheter Cardiovasc Interv. 2006;68(2):319-25. [
MedLine]
16. Dake MD, Miller DC, Mitchell RS, Semba CP, Moore KA, Sakai T, et al. The "first generation" of endovascular stentgrafts for patients with aneurysms of the descending thoracic aorta. J Thorac Cardiovasc Surg. 1998;116(5):689-703. [
MedLine]
17. Estrera Al, Miller CC 3rd, Safi HJ, Goodrick JS, Keyhani A, Porat EE, et al. Outcomes of medical management of acute type B aortic dissection. Circulation. 2006;114(1 Suppl):I384-9. [
MedLine]
18. Onitsuka S, Akashi H, Tayama K, Okazaki T, Ishihara K, Hiromatsu S, et al. Long-term outcome and prognostic predictors of medically treated acute type B aortic dissections. Ann Thorac Surg. 2004;78(4):1268-73. [
MedLine]
19. Bernard Y, Zimmermann H, Chocron S, Litzler JF, Kastler B, Etievent JP, et al. False lumen patency as a predictor of late outcome in aortic dissection. Am J Cardiol. 2001;87(12):1378-82. [
MedLine]
20. Nienaber CA, Rehders TC, Ince H. Interventional strategies for treatment of aortic dissection. J Cardiovasc Surg (Torino). 2006;47(5):487-96. [
MedLine]
21. Eggebrecht H, Nienaber CA, Neuhäuser M, Baumgart D, Kische S, Schmermund A, et al. Endovascular stent-graft placement in aortic dissection: a meta-analysis. Eur Heart J. 2006;27(4):489-98. [
MedLine]
22. Böckler D, Schumacher H, Ganten M, von Tengg-Kobligk H, Schwarzbach M, Fink C, et al. Complications after endovascular repair of acute symptomatic and chronic expanding Stanford type B aortic dissections. J Thorac Cardiovasc Surg. 2006;132(2):361-8. [
MedLine]
23. Resch TA, Delle M, Falkenberg M, Ivancev K, Konrad P, Larzon T, et al. Remodelling of the thoracic aorta after stent grafting of type B dissection: a Swedish multicenter study. J Cardiovasc Surg (Torino). 2006;47(5):503-8. [
MedLine]
24. Won JY, Lee DY, Shim WH, Chang BC, Park SI, Yoon CS, et al. Elective endovascular treatment of descending thoracic aortic aneurysms and chronic dissections with stent-grafts. J Vasc Interv Radiol. 2001;12(5):575-82. [
MedLine]
25. Akutsu K, Nejima J, Kiuchi K, Sasaki K, Ochi M, Tanaka K, et al. Effects of the patent false lumen on the long-term outcome of type B acute aortic dissection. Eur J Cardiothorac Surg. 2004;26(2):359-66. [
MedLine]





 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license