INTRODUCTION
Over the last few years, great interest has been paid to the study of postoperative morbidities of heart surgery due to the decline in mortality rates. Thus, one of the areas that most calls the attention is the impact of these procedures on the brain and cognitive function with serious clinical and financial implications.
In the setting of cardiovascular surgery, since the introduction of the cardiopulmonary bypass (CPB) five decades ago, reports that various patients developed some type of neurological sequel started to emerge. These sequels included from cognitive impairment to fatal strokes [1], with incidences varying from 20 to 83% [2-4] and from 1.5 to 5.2% [5,6], respectively. Hence, these are common consequences causing high costs to the public health system.
As can be seen, there is a great difference among the results published on this subject. This is mainly due to the designs of studies (prospective or retrospective), to open heart or extracardiac surgery, the presence of comorbidities, to the method and time of evaluation of the type and degree of neurological disturbances [7-9]. Thus, among the most commonly studied procedures are coronary artery bypass grafting (CABG).
Impairment of memory, concentration, language, comprehension and social integration, characterizing the so-called cognitive dysfunction (CD), are some of the most frequently affected areas in surgical interventions, situations that can occur from days to months after the surgery and remain for the rest of the life of the patient [1]. Many of these cognitive changes are temporary with resolution in between six weeks and six months after the procedure thereby minimizing medical concern of the importance of transitory cognitive deficits on the quality of life of patients [10].
The main etiology of postoperative CD remains unknown, thus supporting the hypothesis that it is a multifactorial problem [1,8,10,11]. Among the involved risk factors are pre-operative (age, education, previous diseases), intra-operative (number of emboli, duration of the procedure, arterial pressure, temperature, etc.) and post-operative (temperature) [1]. Additionally, some researchers have reported a genetic influence on the surgical outcome, in particular related to the presence of the apolipoprotein E e4allele [12-17].
The aim of this review article is to discuss the main factors involved in neurological lesions after heart surgery and to report the advances of pharmacotherapy in the protection of future neurological outcomes.
Pre-operative factors
a) Age
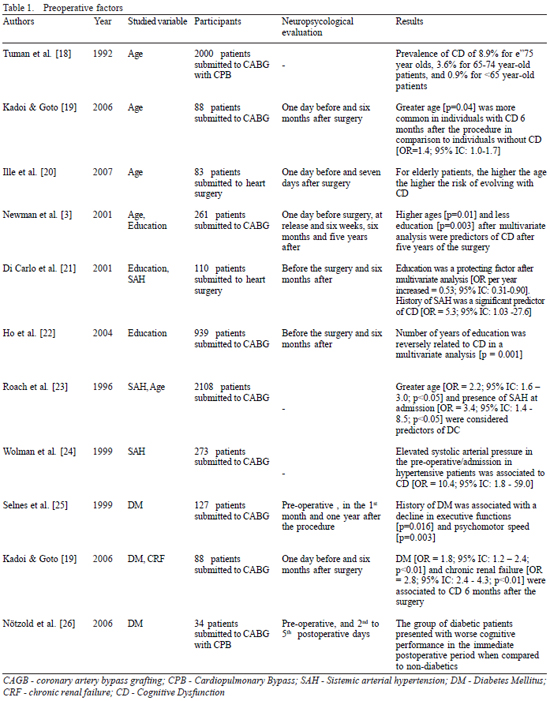
Age is the least controversial demographic risk factor in CD, with a proven relationship with the increase in age (Table 1). However the mechanism by which greater ages are related to postoperative CD remains unknown. Progressive atherosclerosis linked to silent cardiovascular disease and factors intrinsically related to the risk of embolization seem to be the most acceptable explanation for CD to be associated to the increase in age [4,10]. Moreover, older individuals are predisposed to present alterations in the vasculature and auto-regulation of cerebral blood flow [23], an abnormal response to pharmaceutical agents [21] and a natural reduction in the cognitive function which associated with a small postoperative cognitive impairment may cause a significant impact on the quality of life [10].
b) Education
Another pre-operative factor that should be mentioned in the pathogenesis of CD is the level of education. The protective effect of the number of school years, according to some authors (Table 1), seems similar to that suggested in recent studies on Alzheimer's disease, although the mechanism by which this is possible requires further elucidation [24]. It is still not clearly known how a greater number of years of study implies a cognitive reserve, an improvement in evaluation abilities or an increase in the neuronal homeostasis, situations which make the patient more resistant to neuronal injury [10]. One hypothesis, that may explain this association, is based on the fact that education increases the synaptic density in the neocortex, increasing the neuronal communication and minimizing the signs of cognitive and functional impairment [27].
c) Previous diseases
Similar to age and education, the history of comorbidities such as diabetes mellitus (DM), systemic arterial hypertension (SAH) and chronic renal failure (CRF) are factors that are implicated in postoperative neural outcomes.
The history of diabetes mellitus has received the most attention as is seen in the publications listed in Table 1. A possible mechanism which explains this association is the fact that diabetic individuals have an altered auto-regulation of cerebral blood flow, characterized by a greater extraction of oxygen during CPB [19,22] and consequently, reduced availability to the brain. Similarly, SAH and anti-hypertension therapy may be related to the cognitive outcome (Table 1), a reflection of the impaired self-regulation of the cerebral blood flow, hardening of the small cerebral arteries and generalized atherosclerotic disease existent in hypertensive individuals.
Intra-operative factors
a) Embolization
The formation of emboli, whose genesis would be the atheroma of the aortic wall, aggregated platelets, air bubbles originating from the oxygenator and the heart chambers may be the main cause of encephalic injury and the deterioration of pre-existent lesions [8]. These are subdivided in micro and macroemboli, with the former being more relevant in the emergence of CD.
Among the types of emboli, gaseous microemboli are the most probable source of CD, an event which is difficult to diagnose in the intra-operative period. There are three main causes for the genesis of microbubbles: the oxygenator itself, the cooling process of gasses which alters their solubility with predisposition to the formation of bubbles in the blood stream and the opening of heart chambers during the surgical procedure [8].
The understanding that emboli occurring during CPB may cause CD enables the adoption of techniques such as filtering and processing of blood thereby reducing the risk of injury caused by this embolic phenomenon. However, the possible beneficial effects of these new techniques were not confirmed in the results of Rubens et al. [28], which did not show differences in the incidence of CD in individuals submitted to CPB with the processing of blood or not, thus demonstrating the necessity of a continuous evolution of technology.
b) Duration of the surgical procedure
The hypothesis that CPB and the duration of the surgical procedure are related to greater microvascular obstructions by emboli suggests a relationship between these factors and the evolution to CD (Table 2).
In a recent review article, Hogue et al. [39] discussed the impact of CPB on the development of postoperative CD and stressed the importance of recent advances in technology linked to this procedure in the prevention of new neurological events. Hence, the continuous perfecting of the CPB circuit has become fundamental to diminish the migration of emboli, and so significantly collaborate to the reduction in the incidence of CD.
c) Arterial pressure
The importance of the mean arterial pressure (MAP) during the surgery is also the subject of research (Table 2), with intra-operative hypertension and consequent hypoperfusion potentially causing neurological impairment [4]. It is probable that an area of the cerebral irrigated by an occluded artery is exposed to hypoperfusion if the system of collateral arteries are affected by systemic hypotension [40], thereby culminating in an unfavorable cognitive outcome.
However, there are works that do not support this hypothesis. This disparity may be due to the age of the participants of the studies [31]. Great attention should be paid to maintain an adequate MAP in elderly patients as they are more susceptible to brain lesions.
d) Inflammation
Another intra-operative factor that may contribute to neurological lesions is the inflammatory response. It is well known that exposure of blood to the surfaces of the CPB circuit triggers a systemic inflammatory response [40]. In areas affected by hypoperfusion, the exposure of blood to non-endothelial surfaces activates the coagulation cascade and the fibrinolytic and complement systems, as well as favoring the release of free radicals, factors that cause deterioration of pre-existent lesions [8].
The release of inflammatory mediators due to CPB seems to be temperature-dependent, with reductions in the inflammatory response in hypothermic CPB. Hence, a better understanding of the factors involved in CPB and the adoption of therapeutic interventions with the aim of attenuating the inflammatory response may be strategically useful to diminish cerebral lesions [1].
e) Hyperglycemia
Another factor common in heart surgeries is hyperglycemia (blood sugar > 200 mg/dL), a phenomenon with neurological consequences, although not totally understood, that may greatly influence the cerebral metabolism [34]. Initially, high levels of blood sugar lead to an increase in the availability of substrate for the production of lactate in the anaerobic metabolism, a common phenomenon during ischemic processes. Consequently, the resulting acidosis interferes in glycolysis, proteic synthesis, homeostasis, enzymatic functions and in other cell processes [41], which lead to an expansion of the ischemic area [42]. Additionally, there is evidence that link hyperglycemia to an increase in the release of excitatory amino acids, a greater inflammatory response and an increase in the production of corticosteroids, facts that allied to factors described below may contribute to a higher incidence of postoperative CD [34].
F) Temperature: hyperthermia
The effect of temperature during and after CPB on the postoperative cognitive result remains of great interest. With the adverse effects caused by hyperthermia, the significant influence of temperature on the cerebral response to existing damage seems to be of great value.
Several mechanisms may explain the role of hyperthermia and its influence on the brain [43]. Sternau et al. [44], in an experimental study, demonstrated that the release of neurotransmitters in toxic amounts is accentuated by hyperthermia. Similarly, hyperthermia, when compared to normothermia, is associated to a higher release of free radicals [45], an exaggerated increase in the hematoencephalic barrier permeability [46] and enlargement of the ischemic areas by increasing ischemic depolarization [47]. Apart from these effects identified in experimental models, hyperthermia after strokes is associated to an increase in the area of infarction, thus contributing to higher morbidity and mortality rates [43].
Among the neuroprotector factors, hypothermia is the only one that reduces the energy consumption, which is directly related to the maintenance of cell integrity. Thus, this improves the cerebral and myocardial tolerance to the ischemic process [48]. In moderate hypothermia, self-regulation of the cerebral blood flow is intimately correlated to the cerebral metabolic rate, a condition not seen in situations of extreme hypothermia [4]. However, the beneficial effects of maintaining low temperatures are still controversial and there are disadvantages according to some authors (Table 2), with the necessity of rewarming to a normothermic temperature being the most important.
Postoperative factors
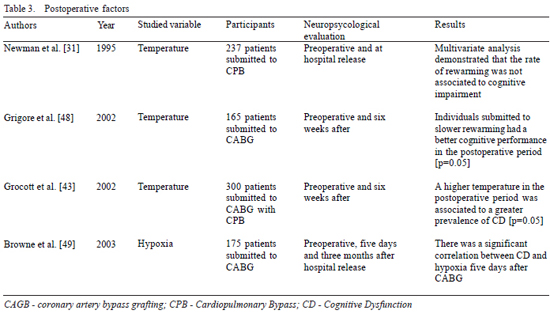
Among the postoperative factors related to CD, studies in respect to hypoxia and the CPB rewarming temperature are highlighted [31,43,48,49]. Browne et al. [49] found that postoperative hypoxia is a risk factor for the development of early CD, a fact confirmed by Hopkins et al. [50], who demonstrated, in individuals submitted to cerebral hypoxia, alterations in the structure of the hippocampus associated to CD. However, these findings were not confirmed by Moller et al. [51], who, on excluding individuals submitted to heart surgery from their work, did not evaluate an important factor related to CD, that is CPB and the consequent postoperative hypoxia. Hence, clearer evidence is necessary to elucidate the role of this condition in the development of CD.
On the other hand, according to what was discussed for the intra-operative factors, the most important adverse effect of the maintenance of low temperatures in CPB is the necessity of rewarming. During rewarming from hypothermic CPB, there may be an overload of cerebral temperature due to the aggressive rewarming process aimed at reducing the time of CPB and of the surgical procedure [40]. Moreover, the speed of rewarming is directly related to jugular desaturation [52], which in turn is associated to cognitive performance [53]. Another important fact in the pathogenesis of cerebral damage is the speed at which the rewarming rate is established, a condition related to the development of CD at a very early stage in the postoperative period (Table 3).
Genetic factors
Although a large number of factors may predict the risk of CD after surgical procedures, these factors contribute to about 10% to 40% of the analyzed models, findings that indicate that other factors may influence impairment [10]. Among the possible genetic polymorphisms that may influence postoperative CD, special attention should be paid to the e4 allele of apolipoprotein E (apo e4). This polymorphism is well known as a risk factor for Alzheimer's disease and for neurodegenerative disorders [54]. In an analysis associating the presence of apo e4 with CD, the results are controversial (Table 4) so other etiological factors associated to the development of CD should be included in studies.

Because of this, several other genetic factors have been associated to CD, although many of these more recent studies are limited either due to small sample sizes or because they analyze genes in isolation (instead of several genes), or by the methodology employed [55]. Another studied polymorphism is the phospholipases A2, which, when present, is associated to lower scores in mental state evaluation examinations [56].
Summed to the aforementioned genetic polymorphisms, the genetic influence of the inflammatory response to CPB has also been stressed as in the cases of interleukin-6, C-reactive protein (CRP) and tumoral necrosis factor-alpha, with the study of these variants being important not only as they associate polymorphisms to an increased inflammatory response but as they link, consequent to this, inflammatory response to side effects related to heart surgery [55]. Hence, a recent publication by Mathew et al. [57] deserves special attention. In this work 37 polymorphisms of simple nucleotides (SNPs) were genotyped for 513 patients submitted to CABG with CPB. The association between these SNPs and cognitive impairment was tested six months after surgery by multiple logistic regression adjusted by age, education, basal cognitive level and populational structure. A significant relationship was obtained among carriers of lower alleles of CRP and a reduction of risk for CD (Odds Ratio = 0.37; 95%CI: 0.16 - 0.78; p-value = 0.013). In carriers of these lower alleles, the pre-operative levels of CRP were lower, ratifying biological support for the observed allelic association.
With advances in technology, the simultaneous investigations of several genes has become more scientifically and economically viable enabling the establishment of how a genetic factor is capable of affecting outcomes.
Pharmacotherapy protection
With knowledge of the risk factors, it is possible to predict those individuals who have a greater chance of evolving with CD and so institute protective measures thereby reducing sequels and avoiding irreversible brain injury. Neuroprotection therapy aims at minimizing the activation of toxic processes and to increase the endogen mechanisms of protection [1].
Initially, effort was focused on anesthetics (thiopental and propofol), based on the hypothesis that a reduced cerebral metabolic demand would provide greater resistance to ischemic events [39]. However, the results were not very satisfactory [58,59]. Other clinical trials had promising results [60-62], suggesting that pharmacological agents, such as remacemide, a glutamate neurotransmitter antagonist (released during the ischemic process and responsible for the rapid inflow of calcium to cells) and lidocaine (at antiarrhythmic doses) may be useful in neuroprotection therapy (Table 5).
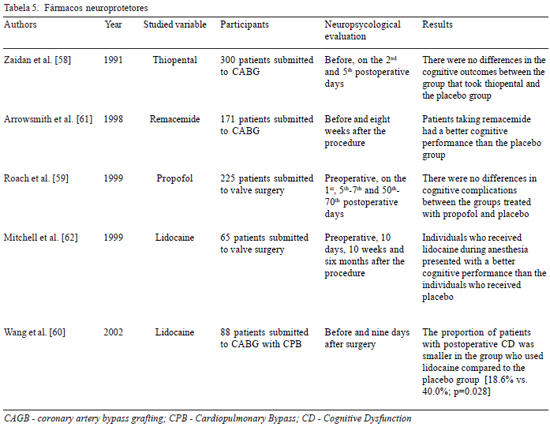
With the exception of some anesthetics, many of the neuroprotecting agents studied in heart surgery were originally developed for the treatment of strokes [4]. Unhappily, the divergence of factors related to brain damage implicate a difficulty in their utilization and, in most cases, the results are disappointing. A possible explanation for these results, apart from the limited sample size, is that the treatment is directed to a specific target, in detriment to a much more complex process which occurs simultaneously.
CONCLUSION
Although heart surgery procedures have gone through major technological changes in recent decades, postoperative cognitive dysfunction remains a common event and causes severe lesions characterized by varying degrees of cognitive loss in a significant number of individuals. Despite of many studies, the physiopathology of these neurological events has not been clearly determined yet, and so the presence of pre-operative, intra-operative and postoperative risk factors as well as a genetic basis is implicated in this disease. Another important observation to be considered is the lack of definition in respect to the ideal pharmacological agent to reduce the incidence of cognitive dysfunction, which suggests the necessity of further studies in this area because of the highly significant number of heart surgeries.
In respect to the most severe events (for example strokes), although they are not the focus of this review article, some brief considerations should be mentioned. The elucidation of the exact physiopathologic mechanism of postoperative cognitive dysfunction helps us to understand factors related to the occurrence of more serious events, as well as how the development of pharmacotherapy to protect against cognitive dysfunction may also contribute to the development of prophylactic measures in the development of strokes.
In conclusion, a continuous process of perfecting tools and techniques utilized during surgical procedures is necessary, thereby contributing to less aggression to the organism of the patient and a progressive reduction in the incidence of neurological complications. Hence, the association of prophylactic measures and a better understanding of the risk factors associated to postoperative cognitive dysfunction will lead to surgical interventions with lower incidences of complications and consequent reductions in hospital costs and improvements in the quality of life of patients.
REFERENCES
1. Gao L, Taha R, Gauvin D, Othmen LB, Wang Y, Blaise G. Postoperative cognitive dysfunction after cardiac surgery. Chest. 2005;128(5):3664-70. [
MedLine]
2. Hornick P, Smith PL, Taylor KM. Cerebral complications after coronary bypass grafting. Curr Opin Cardiol. 1994;9(6):670-9. [
MedLine]
3. Newman MF, Kirchner JL, Phillips-Bute B, Gaver V, Grocott H, Jones RH, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344(6):395-402. [
MedLine]
4. Arrowsmith JE, Grocott HP, Reves JG, Newman MF. Central nervous system complications of cardiac surgery. Br J Anesth. 2000;84(3):378-93.
5. McKhann GM, Goldsborough MA, Borowicz LM Jr, Selnes OA, Mellits ED, Enger C, et al. Cognitive outcome after coronary artery bypass: a one-year prospective study. Ann Thorac Surg. 1997;63(2):510-5. [
MedLine]
6. McKhann GM, Goldsborough MA, Borowicz LM Jr, Mellits ED, Brookmeyer R, Quaskey SA, et al. Predictors of stroke risk in coronary artery bypass patients. Ann Thorac Surg. 1997;63(2):516-21. [
MedLine]
7. Ricksten SE. Cerebral dysfunction after cardiac surgery: are we moving forward? Curr Opin Anaesthesiol. 2000;13(1):15-9. [
MedLine]
8. Lelis RGB, Auler Jr JOC. Lesão neurológica em cirurgia cardíaca: aspectos fisiopatológicos. Rev Bras Anestesiol. 2004;54(4):607-17.
9. Mahanna EP, Blumenthal JA, White WD, Croughwell ND, Clancy CP, Smith LR, et al. Defining neuropsychological dysfunction after coronary artery bypass grafting. Ann Thorac Surg. 1996;61(5):1342-7. [
MedLine]
10. Kadoi Y, Goto F. Factors associated with postoperative cognitive dysfunction in patients undergoing cardiac surgery. Surg Today. 2006;36(12):1053-7. [
MedLine]
11. Newman S, Stygall J. Changes in cognition following cardiac surgery. Heart. 1999;82(5): 541-2. [
MedLine]
12. Tagarakis GI, Tsolaki-Tagaraki F, Tsolaki M, Diegeler A, Tsilimingas NB, Papassotiropoulos A. The role of apolipoprotein E in cognitive decline and delirium after bypass heart operations. Am J Alzheimers Dis Other Demen. 2007;22(3):223-8. [
MedLine]
13. Lelis RG, Krieger JE, Pereira AC, Schmidt AP, Carmona MJ, Oliveira SA, et al. Apolipoprotein E4 genotype increases the risk of postoperative cognitive dysfunction in patients undergoing coronary artery bypass graft surgery. J Cardiovasc Surg (Torino). 2006;47(4):451-6. [
MedLine]
14. Tardiff BE, Newman MF, Saunders AM, Strittmatter WJ, Blumenthal JA, White WD, et al. Preliminary report of a genetic basis for cognitive decline after cardiac operations. The Neurologic Outcome Research Group of the Duke Heart Center. Ann Thorac Surg. 1997;64(3):715-20. [
MedLine]
15. Steed L, Kong R, Stygall J, Acharya J, Bolla M, Harrison MJ, et al. The role of apolipoprotein E in cognitive decline after cardiac operation. Ann Thorac Surg. 2001;71(3):823-6. [
MedLine]
16. Robson MJ, Alston RP, Andrews PJ, Wenham PR, Souter MJ, Deary IJ. Apolipoprotein E and neurocognitive outcome from coronary artery surgery. J Neurol Neurosurg Psychiatry. 2002;72(5):675-6. [
MedLine]
17. Askar FZ, Cetin HY, Kumral E, Cetin O, Acarer A, Kosova B, et al. Apolipoprotein E epsilon4 allele and neurobehavioral status after on-pump coronary artery bypass grafting. J Card Surg. 2005;20(5):501-5. [
MedLine]
18. Tuman KJ, McCarthy RJ, Najafi H, Ivankovich AD. Differential effects of advanced age on neurologic and cardiac risks of coronary artery operations. J Thorac Cardiovasc Surg. 1992;104(6):1510-7. [
MedLine]
19. Kadoi Y, Goto F. Factors associated with postoperative cognitive dysfunction in patients undergoing cardiac surgery. Surg Today. 2006;36(12):1053-7. [
MedLine]
20. Ille R, Lahousen T, Schweiger S, Hofmann P, Kapfhammer HP. Influence of patient-related and surgery-related risk factors on cognitive performance, emotional state, and convalescence after cardiac surgery. Cardiovasc Revasc Med. 2007;8(3):166-9. [
MedLine]
21. Di Carlo A, Perna AM, Pantoni L, Basile AM, Bonacchi M, Pracucci G, et al. Clinically relevant cognitive impairment after cardiac surgery: a 6-month follow-up study. J Neurol Sci. 2001;188(1-2):85-93. [
MedLine]
22. Ho PM, Arciniegas DB, Grigsby J, McCarthy M Jr, McDonald GO, Moritz TE, et al. Predictors of cognitive decline following coronary artery bypass graft surgery. Ann Thorac Surg. 2004;77(2):597-603. [
MedLine]
23. Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med. 1996;335(25):1857-63. [
MedLine]
24. Wolman RL, Nussmeier NA, Aggarwal A, Kanchuger MS, Roach GW, Newman MF, et al. Cerebral injury after cardiac surgery: identification of a group at extraordinary risk. Multicenter Study of Perioperative Ischemia Research Group (McSPI) and the Ischemia Research Education Foundation (IREF) Investigators. Stroke. 1999;30(3):514-22. [
MedLine]
25. Selnes OA, Goldsborough MA, Borowicz LM Jr, Enger C, Quaskey SA, McKhann GM. Determinants of cognitive change after coronary artery bypass surgery: a multifactorial problem. Ann Thorac Surg. 1999;67(6):1669-76. [
MedLine]
26. Nötzold A, Michel K, Khattab AA, Sievers HH, Hüppe M. Diabetes mellitus increases adverse neurocognitive outcome after coronary artery bypass grafting surgery. Thorac Cardiovasc Surg. 2006;54(5):307-12. [
MedLine]
27. Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology. 1993;43(1):13-20. [
MedLine]
28. Rubens FD, Boodhwani M, Mesana T, Wozny D, Wells G, Nathan HJ. The cardiotomy trial: a randomized, double-blind study to assess the effect of processing of shed blood during cardiopulmonary bypass on transfusion and neurocognitive function. Circulation. 2007;116(11 Suppl):I89-97. [
MedLine]
29. Stroobant N, Van Nooten G, Van Belleghem Y, Vingerhoets G. Relation between neurocognitive impairment, embolic load, and cerebrovascular reactivity following on- and off-pump coronary artery bypass grafting. Chest. 2005;127(6):1967-76. [
MedLine]
30. Yin YQ, Luo AL, Guo XY, Li LH, Huang YG. Postoperative neuropsychological change and its underlying mechanism in patients undergoing coronary artery bypass grafting. Chin Med J (Engl). 2007;120(22):1951-7. [
MedLine]
31. Newman MF, Kramer D, Croughwell ND, Sanderson I, Blumenthal JA, White WD, et al. Differential age effects of mean arterial pressure and rewarming on cognitive dysfunction after cardiac surgery. Anesth Analg. 1995;81(2):236-42. [
MedLine]
32. Gold JP, Charlson ME, Williams-Russo P, Szatrowski TP, Peterson JC, Pirraglia PA. Improvement of outcomes after coronary artery bypass. A randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110(5):1302-11.
33. Gottesman RF, Hillis AE, Grega MA, Borowicz LM Jr, Selnes OA, Baumgartner WA, et al. Early postoperative cognitive dysfunction and blood pressure during coronary artery bypass graft operation. Arch Neurol. 2007;64(8):1111-4. [
MedLine]
34. Puskas F, Grocott HP, White WD, Mathew JP, Newman MF, Bar-Yosef S. Intraoperative hyperglycemia and cognitive decline after CABG. Ann Thorac Surg. 2007;84(5):1467-73. [
MedLine]
35. Grigore AM, Mathew J, Grocott HP, Reves JG, Blumenthal JA, White WD, et al. Prospective randomized trial of normothermic versus hypothermic cardiopulmonary bypass on cognitive function after coronary artery bypass graft surgery. Anesthesiology. 2001;95(5):1110-9. [
MedLine]
36. Boodhwani M, Rubens FD, Wozny D, Rodriguez R, Alsefaou A, Hendry PJ, et al. Predictors of early neurocognitive deficits in low-risk patients undergoing on-pump coronary artery bypass surgery. Circulation. 2006;114(1 Suppl):I461-6. [
MedLine]
37. Boodhwani M, Rubens F, Wozny D, Rodriguez R, Nathan HJ. Effects of sustained mild hypothermia on neurocognitive function after coronary artery bypass surgery: a randomized, double-blind study. J Thorac Cardiovasc Surg. 2007;134(6):1443-50.
38. Nathan HJ, Rodriguez R, Wozny D, Dupuis JY, Rubens FD, Bryson GL, et al. Neuroprotective effect of mild hypothermia in patients undergoing coronary artery surgery with cardiopulmonary bypass: five-year follow-up of randomized trial. J Thorac Cardiovasc Surg. 2007;133(5):1206-11. [
MedLine]
39. Hogue CW Jr, Palin CA, Arrowsmith JE. Cardiopulmonary bypass management and neurologic outcomes: an evidence-based appraisal of current practices. Anesth Analg. 2006;103(1):21-37. [
MedLine]
40. Grocott HP, Homi HM, Puskas F. Cognitive dysfunction after cardiac surgery: revisiting etiology. Semin Cardiothorac Vasc Anesth. 2005;9(2):123-9. [
MedLine]
41. Grocott HP, Yoshitani K. Neuroprotection during cardiac surgery. J Anesth. 2007;21(3):367-77. [
MedLine]
42. Anderson RE, Tan WK, Martin HS, Meyer FB. Effects of glucose and PaO2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic penumbra. Stroke. 1999;30(1):160-70. [
MedLine]
43. Grocott HP, Mackensen GB, Grigore AM, Mathew J, Reves JG, Phillips-Bute B, et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke. 2002;33(2):537-41. [
MedLine]
44. Sternau L, Globus MT, Dietrich W, Martinez E, Busto R, Ginsberg M. Ischemia-induced neurotransmitter release: effects of mild intraischemic hyperthermia. In: Globus MT, Dietrich W, eds. The role of neurotransmitters in brain injury. New York, NY: Plenum Press;1992. p.33-8.
45. Globus M, Busto R, Lin B, Schnippering H, Ginsberg MD. Detection of free radical activity during transient global ischemia and recirculation: effects of intraischemic brain temperature modulation. J Neurochem. 1995;65(3):1250-6. [
MedLine]
46. Dietrich WD, Halley M, Valdes I, Busto R. Interrelationships between increased vascular permeability and acute neuronal damage following temperature-controlled brain ischemia in rats. Acta Neuropathol. 1991;81(6):615-25. [
MedLine]
47. Chen Q, Chopp M, Bodzin G, Chen H. Temperature modulation of cerebral depolarization during focal cerebral ischemia in rats: correlation with ischemic injury. J Cereb Blood Flow Metab. 1993;13(3):389-94. [
MedLine]
48. Grigore AM, Grocott HP, Mathew JP, Phillips-Bute B, Stanley TO, Butler A, et al. The rewarming rate and increased peak temperature after neurocognitive outocome after cardiac surgery. Anesth Analg. 2002;94(1):4-10. [
MedLine]
49. Browne SM, Halligan PW, Wade DT, Taggart DP. Postoperative hypoxia is a contributory factor to cognitive impairment after cardiac surgery. J Thorac Cardiovasc Surg. 2003;126(4):1061-4. [
MedLine]
50. Hopkins RO, Kesner RP, Goldstein M. Item and order recognition memory in subjects with hypoxic brain injury. Brain Cogn. 1995;27(2):180-201. [
MedLine]
51. Moller JT, Cluitmans P, Rasmussen LS, Houx P, Rasmussen H, Canet J, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351(9106):857-61. [
MedLine]
52. Chen CS, Leu BK, Liu K. Detection of cerebral desaturation during cardiopulmonary bypass by cerebral oximetry. Acta Anaesthesiol Sin. 1996;34(4):173-8. [
MedLine]
53. Croughwell ND, Newman MF, Blumenthal JA, White WD, Lewis JB, Frasco PE, et al. Jugular bulb saturation and cognitive dysfunction after cardiopulmonary bypass. Ann Thorac Surg. 1994; 58 (6):1702-8. [
MedLine]
54. Huang W, Qiu C, von Strauss E, Winblad B, Fratiglioni L. APOE genotype, family history of dementia, and Alzheimer disease risk: a 6-year follow-up study. Arch Neurol. 2004;61(12):1930-4. [
MedLine]
55. Grocott HP. Genetic influences on cerebral outcome after cardiac surgery. Semin Cardiothorac Vasc Anesth. 2006;10(4):291-6. [
MedLine]
56. Mathew JP, Rinder CS, Howe JG, Fontes M, Crouch J, Newman MF, et al. Platelet PlA2 polymorphism enhances risk of neurocognitive decline after cardiopulmonary bypass. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Ann Thorac Surg. 2001;71(2):663-6. [
MedLine]
57. Mathew JP, Podgoreanu MV, Grocott HP, White WD, Morris RW, Stafford-Smith M, et al. Genetic variants in P-selectin and C-reactive protein influence susceptibility to cognitive decline after cardiac surgery. J Am Coll Cardiol. 2007;49(19):1934-42. [
MedLine]
58. Zaidan JR, Klochany A, Martin WM, Ziegler JS, Harless DM, Andrews RB. Effect of thiopental on neurologic outcome following coronary artery bypass grafting. Anesthesiology. 1991;74(3):406-11. [
MedLine]
59. Roach GW, Newman MF, Murkin JM, Martzke J, Ruskin A, Li J, et al. Ineffectiveness of burst suppression therapy in mitigating perioperative cerebrovascular dysfunction. Multicenter Study of Perioperative Ischemia (McSPI) Research Group. Anesthesiology. 1999;90(5)1255-64. [
MedLine]
60. Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg. 2002;95(5):1134-41.
61. Arrowsmith JE, Harrison MJ, Newman SP, Stygall J, Timberlake N, Pugsley WB. Neuroprotection of the brain during cardiopulmonary bypass: a randomized trial of remacemide during coronary artery bypass in 171 patients. Stroke. 1998;29(11):2357-62. [
MedLine]
62. Mitchell SJ, Pellett O, Gorman DF. Cerebral protection by lidocaine during cardiac operations. Ann Thorac Surg. 1999; 67(4):1117-24. [
MedLine]



 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license