Elaine Soraya Barbosa de Oliveira SeverinoI; Orlando PetrucciII; Karlos Alexandre de Souza VilarinhoIII; Carlos Fernando Ramos LavagnoliIV; Lindemberg da Mota Silveira FilhoIII; Pedro Paulo Martins de OliveiraV; Reinaldo Wilson VieiraVI; Domingo Marcolino BraileVII
DOI: 10.5935/1678-9741.20110045
INTRODUCTION
The mitral valve repair is the procedure of choice in the cause degenerative mitral valve disease, it has lower rate of reoperation, thromboembolism and valve infection, when compared to mitral valve replacement [1]. Rheumatic heart disease is the leading cause of mitral valve disease in the developing world, including Brazil. The mitral valve repair in rheumatic disease is technically more difficult, and the outcomes may be interfered with by new exacerbations. The quality and long-term results in rheumatic mitral valve disease are controversial [1,2].
The aim of this study was to assess factors associated with reoperation and mortality in patients undergoing only conservative procedures in rheumatic mitral valve cause. We aim also to assess the effectiveness of this procedure in this population.
METHODS
After approval by the Research Ethics Committee and registration at National Commission on Ethics in Research (CONEP), we performed a cohort study in patients undergone surgery between January 1994 and December 2005, when there were 700 heart valve surgeries.
The study included patients with rheumatic mitral valve disease submitted to the first procedure in mitral valve repair. Patients with or without tricuspid regurgitation were also included in the study. Exclusion criteria were: procedures associated with mitral valve repair, such as aortic valve replacement or coronary artery bypass grafting. Patients with prior surgery of mitral valve repair were also excluded.
The surgeries were performed by median sternotomy with bicaval cannulation and normothermic cardiopulmonary bypass. Low voolume warm blood cardioplegia was used. The mitral valve was accessed through the left atriotomy through the interatrial sulcus. As intraoperative assessment, we applied the techniques of mitral valve repair, including mitral valve repair with flexible ring (Braile Biomedica®, São José do Rio Preto, Brazil), commissurotomy combined with mitral valve repair or commissurotomy.
During this period, patients underwent surgery by five different surgeons at a single institution. The intraoperative echocardiogram was not performed in all cases.
The demographics of patients, pre- and postoperative echocardiographic assessments, functional class, according to the New York Heart Association (NYHA), and mortality were obtained retrospectively. Follow-up was considered as the last visit at the institution.
Statistical Analysis
Continuous variables were expressed as mean with standard deviation. The discrete variables were described by percentage. We used the Kolmogorov-Smirnov test for the detection of a normal sample, and then used the Student's t test or Wilcoxon test for paired samples when appropriate. Survival and reoperation were assessed by Kaplan-Meier and proportional hazards regression using the Cox stepwise method. We used the software Medcalc 4.0 (Brussels, Belgium). The P value of <0.05 was considered significant.
RESULTS
We obtained data from 104 patients. The mean follow-up was 63 ± 39 months (95% CI 36 to 74 months), ranging from 3 to 130 months. There was predominance of females (78.8%), mean age 32.73 ± 14.74 years. Pure mitral insufficiency was present in 35.7% of patients, pure stenosis in 27.8% and mixed lesions in 36.5%. Functional class III and IV (NYHA) were observed in 65.4% of patients preoperatively. Pre- and postoperative cchocardiographic data are shown in Table 1.
We observed that there was reduction of left atrial size and ejection fraction and functional class improvement, all these parameters was statistically significant.
33 plasties were performed using ring, 21 commissurotomies and 50 commissurotomy and mitral ring. There was no operative mortality. The tricuspid valvuloplasty was performed concomitantly in 12 patients, all with the use of flexible ring (Braile Biomedica ®), according to the technique previously described [4]. There were no deaths until hospital discharge.
In the follow-up, patients free from reoperation with five and 10 years were 91.2 ± 3.4% and 71.1 ± 9.2%, respectively (Figure 1).
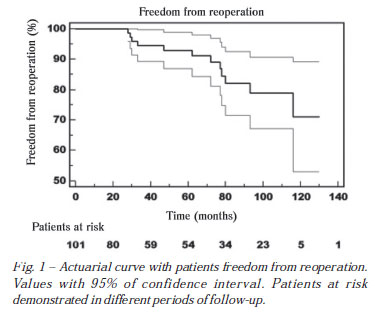
One patient died 90 months after surgery with pulmonary embolism caused by chronic myeloid leukemia. Another patient underwent mitral valve replacement at 77 months, and 11 months after surgery showed thrombosis of the mitral prosthesis. The third patient died of unknown causes.
The survival rate with five and 10 years at follow-up was 99.0 ± 0.1% and 92.1 ± 0.04%, respectively (Figure 2).
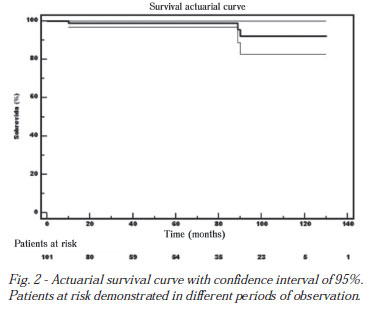
During follow-up, 12 patients underwent reoperation, with a mean interval to reoperation of 61 months, ranging between 27 and 116 months. Reoperated patients had mitral regurgitation (n = 5), mitral stenosis (n = 2), mitral lesion (n = 3), mitral stenosis and aortic regurgitation concomitantly (n = 2).
In regression analysis with the Cox proportional hazards using the stepwise method, were found as independent predictors of reoperation the variables listed in Table 2, with respective odds ratio (OR). In this analysis, we used the clinical and echocardiographic variables.
DISCUSSION
The mitral valve repair is the procedure of choice in patients with mitral regurgitation caused by degenerative disease, where the techniques employed have lower rates of morbidity and mortality and good long-term survival, with most of the patients free of reoperation and/or thromboembolic complications [5]. However, the application of these techniques in rheumatic valve disease, even when feasible by valve morphology, is still controversial in the literature, with controversial results [3,4,6,7].
One of the limiting factors for the repair in rheumatic disease is the evolutionary character of valve degeneration. Duran et al. [5], dividing patients undergoing mitral valve repair by age, observed a greater chance of valve repair in patients younger than 20 years, compared with patients between 21-40 and above 40 years. However, those under the age of 20 years had a higher risk of reoperation when compared to patients with more than 20 years of age. This result was also described in Brazilian study, performed by Pomerantzeff et al. [8], where the cutoff age was 16 years. Another related factor was the moment of surgical indication. The earlier the surgery, the lower the commitment of the valve or subvalvular apparatus, less distortion of ventricular geometry and, consequently, the greater the chances of successful functional repair, and preservation of ventricular function. We obtained an independent predictor of patient age at surgery, ie, the older the patient, increases his chance of reoperation, which makes sense because these patients have their subvalvar already well lesioned, the functional outcome of plasty can be compromised.
Another decisive factor in the choice between repair or valve replacement are the results of the use of prostheses. Yau et al. [10], in a comparative study of repair, metal prostheses and bioprostheses, identified the repair as independent risk factor for longer survival for cardiac death, despite the lower survival free of reoperation for valve repair compared to the use of metallic prostheses. Reoperation was not a risk factor for mortality.
In this study, the mortality rate was zero. One of the factors contributing to this mortality was the fact we have not included plasties combined with other procedures such as aortic valve replacement or coronary artery bypass grafting. We think that these procedures could be an associated factor that hinders any analysis on the evolution of these patients. The aim of this study was to study only the effect of conservative procedures in rheumatic mitral valve.
The results of postoperative echocardiography can demonstrate good results with the repair in the short term, as already shown in other national studies [4]. The presence of mild regurgitation in the postoperative period does not seem to worsen the clinical outcome of these patients [9].
The moderate and severe mitral regurgitation, when detected at follow-up proved to be an independent risk factor for reoperation with an odds ratio of 4.6, ie, following mitral regurgitation increases the risk of reoperation in almost 5 times. This is corroborated by previous study of literature, with patients similar to this study [3,7,8,12,15].
The technique used in our series was described by Braile et al. [2] utilizing a malleable bovine pericardium prosthesis, which is strong and early endothelized without thromboembolic events.
When comparing pre- and postoperative echocardiographic data, considering the systolic and diastolic diameters and left ventricular ejection fraction, we observed that there was a worsening of the ejection fraction. We believe it is an adaptation of the left ventricle after the repair, as the valve became competent.
In this sample, 80% of patients had pure mitral insufficiency or other associated injuries, with the long-term ventricular remodeling. The ejection fraction alone can not lead us to infer a worsening of the patient. This is not confirmed when we assessed the patients' functional class during follow-up, with overall improvement. The assessment of ejection fraction preoperatively may have been overestimated by the presence of mitral regurgitation, which could explain the worsening of the ejection fraction postoperatively. There was significant improvement during follow-up, with concordance with other studies in the literature [3-5].
Most studies reported significant improvement in functional class after valve procedure, either valve repair or prosthesis replacement [3,4,10]. The presence of heart failure postoperatively was another independent risk factor, which makes perfect sense.
Independent factor not previously identified in the literature was the presence of pulmonary hypertension measured by echocardiography preoperatively and is considered moderate when the mean pulmonary artery pressure was measured as greater than 45 mmHg. This factor led to almost twice the risk of reoperation in these patients, this data can serve as a starting point for closer follow-up in such patients.
In relation to valve morphology, few studies were able to identify a type of injury as a predictor, but it seems that the double lesion present behavior worse for late failure of valve repair [13-15]. We could not identify any variable in the anatomy of the valve that was a predictor of reoperation.
Chauvaud et al. [16] in a large series studying 951 patients with mitral regurgitation of rheumatic etiology and follow-up of approximately 25 years, demonstrated probability of being free from reoperation of 55% after ten years of follow-up. In our series, at the end of 10 years of follow-up, the probability of patients being free from reoperation was 70%, but we should note that only 5 patients were at risk during this period of observation. Another important observation is that in the series of Chauvaud et al. patients with pure mitral insufficiency were included, unlike our series, and included patients with mitral stenosis, mitral regurgitation and mitral lesion.
Late mortality in this series was 3.8%, consistent with the literature [4,10]. However, the deaths were not related to heart disease in itself but a result of associated neoplastic illness, one due to a thromboembolic complication after prosthetic valve reoperation, and the last patient, without apparent cause.
The limitations of our study are related to its retrospective nature. Medical treatment was not standardized, it was noted the presence of atrial fibrillation in the pre- and postoperative period.
In conclusion, the results of mitral valve repair in rheumatic patients, when feasible from a technical standpoint and valve morphology, have satisfactory results in the long term, and always appear as an alternative in surgical treatment of this disease. The presence of pulmonary hypertension preoperatively should draw attention to a closer follow-up of these patients due to the long-term risk of reoperation. Older patients with rheumatic valvular heart disease have increased chance of being re-operated when the valve is very committed.
Patients who in the postoperative follow-up present worsening of mitral or functional class must also be followed more often because they have increased risk for reoperation.
REFERENCES
1. Sarris GE, Cahill PD, Hansen DE, Derby GC, Miller DC. Restoration of left ventricular systolic performance after reattachment of the mitral chordae tendineae. The importance of valvular-ventricular interaction. J Thorac Cardiovasc Surg. 1988;95(6):969-79. [MedLine]
2. Braile DM, Ardito RV, Pinto GH, Santos JLV, Zaiantchick M, Souza DRS, et al. Plástica mitral. Rev Bras Cir Cardiovasc. 1990;5(2):86-98. View article
3. David TE, Armstrong S, Sun Z, Daniel L. Late results of mitral valve repais for mitral regurgitation due to degenerative disease. Ann Thorac Surg. 1993;56(1):7-12. [MedLine]
4. Petrucci Junior O, Oliveira PPM, Silveira LM, Passos FM, Vieira RW, Braile DM. Resultados a médio prazo de anuloplastia com órtese maleável de pericárdio bovino na insuficiência mitral reumática. Rev Bras Cir Cardiovasc. 1999;14(2):105-8. View article
5. Duran CM, Gometza B, Saad E. Valve repair in rheumatic mitral disease: an unsolved problem. J Card Surg. 1994;9(2 Suppl):282-5. [MedLine]
6. Pomerantzeff PMA, Brandão CMA, Cauduro P, Puig LB, Grinberg M, Tarasoutchi F, et al. Biopróteses de pericárdio bovino Fisics-Incor: 15 anos. Rev Bras Cir Cardiovasc. 1997;12(4):359-66. View article
7. Gillinov AM, Cosgrove DM, Blackstone EH, Diaz R, Arnold JH, Lytle BW, et al. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg. 1998;116(5):734-43. [MedLine]
8. Pomerantzeff PMA, Brandão CMA, Faber CM, Grinberg M, Cardoso LF, Tarasoutchi F, et al. Plástica da valva mitral em portadores de febre reumática. Rev Bras Cir Cardiovasc. 1998;13(3):211-5. View article
9 . Fix J, Isada L, Cosgrove D, Miller DP, Savage R, Blum J, et al . Do patients with less than 'echo-perfect' results from mitral valve repair by intraoperative echocardiography have a different outcome? Circulation . 1993 ; 88 ( 5 Part 2 ): II39-48 . [MedLine]
10. Yau TM, El-Ghoneimi YA, Armstrong S, Ivanov J, David TE. Mitral valve repair and replacement for rheumatic disease. J Thorac Cardiovasc Surg. 2000;119(1):53-60. [MedLine]
11. Provenzano Junior SC, Sá MPL, Bastos ES, Azevedo JAP, Murad H, Gomes EC, et al. Plastia valvar mitral na doença cardíaca reumática e degeneração mixomatosa: estudo comparativo. Rev Bras Cir Cardiovasc. 2002;17(1):24-34. View article
12. Shuhaiber J, Anderson RJ. Meta-analysis of clinical outcomes following surgical mitral valve repair or replacement. Eur J Cardiothorac Surg. 2007;31(2):267-75. [MedLine]
13. Fernandez J, Joyce DH, Hirschfeld K, Chen C, Laub GW, Adkins MS, et al. Factors affecting mitral valve reoperation in 317 survivors after mitral valve reconstruction. Ann Thorac Surg. 1992;54(3):440-7.
14. Pomerantzeff PM, Brandão CM, Leite Filho OA, Guedes MA, Silva MF, Grinberg M, et al. Mitral valve repair in rheumatic patients with mitral insuficiency: twenty years of techniques and results. Rev Bras Cir Cardiovasc. 2009;24(4):485-9. [MedLine]
15. Kalil RAK, Cunha B, Albrecht AS, Moreno P, Abrahão R, Prates PR, et al. Comparative results of maze procedure for chronic atrial fibrillation in rheumatic and degenerative mitral valve disease. Rev Bras Cir Cardiovasc. 1999;14(3):191-9.
16 . Chauvaud S, Fuzellier JF, Berrebi A, Deloche A, Fabiani JN, Carpentier A . Long-term (29 years) results of reconstructive surgery in rheumatic mitral valve insufficiency . Circulation . 2001 ; 104 ( 12 Suppl 1 ): I-12-5 .
Article receive on Monday, April 4, 2011
 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license