ABSTRACT
Objective: To assess the effects of ischemic postconditioning on left ventricular function in isolated rat hearts. Methods: Twenty-four Wistar rats were used. These hearts underwent perfusion by modified LANGERDORFF method and distributed into three groups: GI - control (n=8); GII - three cycles of postconditioning of 10/10s (n=8); GIII - three cycles of postconditioning of 30/30s (n=8). After a 15min stabilization period, all hearts underwent 20min of global ischemia following 20min of reperfusion. In the times 0 (control), 5, 10, 15 and 20min of reperfusion, the heart rate (HR), the coronary flow (CoF), the systolic pressure, the (+dP/dt max) contractility and (-dP/dt max) velocity of relaxation were measured. Data were analyzed by ANOVA method followed by Tukey's test for differences between groups and P < 0.05 was considered significant. Results: The HR (bpm) decreased in all groups after 20min of reperfusion without statistical differences among them (GI 232.5+ 36.8; GII 241.8+ 46.7; GIII 249.4+ 40.4;P>0.05). The same occurred with the systolic pressure (mmHg) (GI 132.6+49.3; GII 140.8+ 43.1; GIII 112.6+33.2; P>0.05), coronary blood flow (GI 18.5+ 4.6; GII 21.4+ 4.4; GIII 22.1+ 9.0; P>0.05) and -dP/dt max (mmHg) (GI 1490.6+ 512.0; GII 1770.4+ 406.6; GIII 1399.1+ 327.4; P>0.05). The + dP/dt max (mmHg) decreased significantly in all groups except in group II (GI 1409.0+ 415.2, GII 1917.3+ 403.1, GIII 1344.8+355.8) (GII vs GI, P= 0.04; GII vs GIII, P= 0.02). Conclusion: The ischemic postconditioning by three cycles of reperfusion/ischemia of 10/10s demonstrated to be effective for preservation of the myocardial contractility in isolated rat hearts which had undergone 20min of ischemia.
RESUMO
OBJETIVO: Avaliar os efeitos do pós-condicionamento isquêmico na função ventricular esquerda de corações isolados de ratos. MÉTODOS: Corações isolados de 24 ratos Wistar foram submetidos a perfusão pelo método de Langendorff modificado e distribuídos em três grupos: Grupo I - controle (n=8); Grupo II - três ciclos de pós-condicionamento de 10/10s (n=8); Grupo III - três ciclos de pós-condicionamento de 30/30s (n=8). Após estabilização de 15min, os corações foram submetidos a 20min de isquemia e subseqüentes 20min de reperfusão. A freqüência cardíaca (FC), o fluxo coronariano (FCo), a pressão sistólica (PS), a contratilidade (+dP/dt max) e a velocidade de relaxamento (-dP/dt max) miocárdico foram medidas nos tempos 0 (pré-isquemia) e 5, 10, 15 e 20min de reperfusão. Utilizado método estatístico ANOVA com Teste de Tukey para diferenças entre grupos, com significância menor que 0,05 (P< 0,05). RESULTADOS: A FC, em bpm, reduziu em todos os grupos após 20min de reperfusão, sem diferença estatística (GI 232,5 + 36,8; GII 241,8 + 46,7; GIII 249,4 + 40,4; P>0,05). O mesmo ocorreu com a PS, em mmHg, (GI 132,6 + 49,3; GII 140,8 + 43,1; GIII 112,6 + 33,2; P>0,05), FCo, em ml/min, (GI 18,5 + 4,6; GII 21,4 + 4,4; GIII 22,1 + 9,0; P>0,05) e -dP/dt max, em mmHg/s, (GI 1490,6 + 512,0; GII 1770,4 + 406,6; GIII 1399,1 + 327,4, P>0,05). A +dP/dt max, em mmHg/s, reduziu significativamente exceto no Grupo II (GI 1409,0 + 415,2; GII 1917,3 + 403,1; GIII 1344,8 + 355,8), (GII vs. GI, P=0,04) e (GII vs. GIII, P=0,02). CONCLUSÃO: O pós-condicionamento isquêmico com três ciclos de 10/10s de reperfusão/isquemia foi capaz de preservar a contratilidade miocárdica em corações isolados de ratos após 20min de isquemia.
INTRODUCTION
In acute myocardial infarction, restoration of coronary flow, either by thrombolysis or primary angioplasty, is essential to preserve viable myocardium. The size of the infarcted area is a determining factor of the extent of ventricular remodeling and prognosis of patients [1,2]. The early reperfusion is the definitive treatment to reduce the infarcted area, the left ventricular contractile dysfunction and apoptosis. However, reperfusion itself carries the potential risk of worsen the tissue damage in the end of ischemia - a phenomenon known as reperfusion injury [3,4].
In 2003, a phenomenon named ischemic postconditioning was described by Zhao et al. [5]. Such phenomenon was described as brief and repeated episodes of ischemia reperfusion immediately after a period of sustained ischemia, producing less myocardial injury. In their initial study, using
in situ dog hearts, the authors performed a ligation of the anterior interventricular artery for 60 minutes (min), followed by 3 hours (h) of reperfusion (control). Three cycles of reperfusion and ischemia - both with equal length of 30 seconds (s) immediately before 3-hours reperfusion - were used in the postconditioning group, resulting in reduction of 44% of the infarcted area in relation to the control group [5].
The temporal characteristics of postconditioning are very important. In the isolated rat heart, the algorithm of reperfusion and ischemia, both with equal length of 30s, did not reduce the infarcted area [6,7]. In the indexed literature (Medline/Pubmed), functional assessment of isolated rat hearts undergone different algorithms of ischemic postconditioning was not found.
The aim of this study was to assess the effects of ischemic postconditioning on left ventricular function of isolated rat hearts undergone two different time algorithms of reperfusion and ischemia.
METHODS
The protocol was approved by the Ethics Committee of São Francisco de Cardiovascular Foundation, and the animals treated according to ethical principles in Animal Experimentation established by the Brazilian College of Animal Experimentation (COBEA) [8].
We used 24 isolated rat hearts (albino Wistar rats), regardless gender, with weights ranging between 280g and 315g, mean of 296.5 + 4.3g. After inhalation anesthesia with ethilic ether, administration of 500UI of systemic heparin and thoracotomy, the rat hearts were isolated and immediately placed under perfusion with Krebs-Henseleit solution [9], balanced with 95% O
2 and 5% CO
2 and maintained at controlled temperature conditions and perfusion pressure on disposable equipment [10] (Model Comex Ind. & Com Ltda - MG) based on the device used in a pioneering way by Langendorff [11]. Pressure and heart rate record were processed by biomonitor (BESE/Bioengenharia Ltda.-MG, Model HD 073) and printed for analysis and comparative study on dot matrix printer (Epson LQ-1070). The coronary flow was measured by the minute volume from cardiac cavities and collected in graduated glass bottle.
The hearts were divided into three groups: GI - Control (n=8) - ischemia for 20 min, by interrupting the coronary perfusion, and reperfusion for 20 min; GII - postconditioning 10/10s (n=8) - ischemia for 20min, followed by three cycles of reperfusion and ischemia, both with equal length of 10s, and reperfusion for 20min; GIII - postconditioning 30/30s (n=8) - ischemia for 20min, followed by three cycles of ischemia and reperfusion , both with equal length of 30s, and reperfusion for 20min (Figure 1).
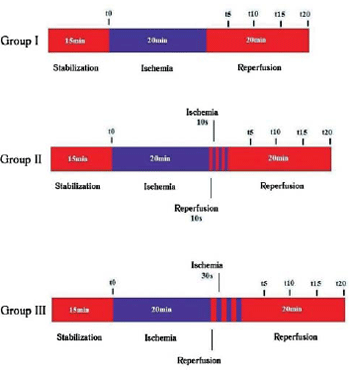
Fig. 1 - Algorithm of the different groups studied. I, control group II, postconditioning 10/10s, III, post-conditioning 30/30s. t0, time immediately before the period of ischemia, t5, t10, t15, t20, corresponding to 5min, 10min, 15min and 20min of reperfusion, respectively
After placing of the hearts in perfusion chamber and before any other procedure, all hearts were maintained for 15 minutes to stabilize their functions. All hearts underwent the same initial time of 20 minutes of ischemia before reperfusion. For analysis of the variables studied, the time control (t0) was considered as that time immediately before the period of ischemia. After the period of ischemia, the variables were recorded with 5min (t
5), 10min (T
10), 15min (T
15) and 20min (T
20) of reperfusion, respectively.
Heart rate (HR), coronary flow (CF), systolic (SP) and diastolic (DP) pressure were recorded. The diastolic pressure was adjusted and maintained at 5±2 mmHg, except during periods of induced ischemia, that was maintained at zero. The effects on myocardial contractility and left ventricle relaxation was measured by calculating the first positive temporal derivative (+dP/dt
max) [12] and negative (-dP/dt
max) [13] of the left ventricular pressure, from measures obtained from the ventricular pressure curves, according to the method described by Gottschall [14].
Data were submitted to the Shapiro-Wilk test for normality, and, after that, to the parametric analysis by the unidirectional ANOVA method combined with Tukey test. The results were expressed as mean ± standard deviation and considered the significance level of less than 5% (P <0.05). We used the software for statistical calculations StatsDirect version 2.6.5.
RESULTS
The hearts weighed between 1.1 g and 1.6 g with a mean of 1.3 + 0.02 g. There were no significant differences between the heart weights in different groups (P=0.68).
The HR decreased in all groups after 20min of reperfusion, with no statistical difference between them (GI 232.5 ± 36.8, GII 241.8 ± 46.7, GIII 249.4 ± 40.4, P> 0.05) (Table 1). The same happened with the CF (GI 18.5 ± 4.6, GII 21.4 ± 4.4, GIII 22.1 ± 9.0, P>0.05), with the SP (GI 132.6 ± 49.3, GII 140.8 ± 43.1, GIII 112.6 + 33.2, P>0.05), and the -dP/dt
max (GI 1490.6 ± 512.0, GII 1770.4 ± 406.6, GIII 1399.1 ± 327.4, P> 0.05) (Tables 2, 3 and 4).
The myocardial contractility progressively decreased in GI up to 20min of reperfusion t
0= 1947.9 ± 311.3; t
5= 1659.8 ± 421.3; t
10= 1599.5 ± 447.1; t
15= 1526 ± 424.9; t
20= 1409 ± 415.2). In the postconditioning group 10/10s (GII), there was decrease in +dP/dt max up to t
5 and subsequent recovery (t
0= 1994.1 ± 460.7; t
5= 1606.5 ± 310.8; t
10= 1780.1 ± 401.9; t
15= 1815.4 ± 521.5; t
20= 1917.3 ± 403.1). In GIII, the decrease of +dP/dt max occurred at t
5 with stable maintenance of loss up to t
20 (t
0= 1673,6 ± 250; t
5= 1439,1 ± 395,8; t
10= 1521,9 ± 435,5; t
15= 1492,1 ± 437,6; t
20= 1344,8 ± 355,8). In the comparison between groups, the changes noted in myocardial contractility showed significant differences in time t20 (GI 1409.0 ± 415.2~ GII 1917 ± 403.1; GIII 1344.8 + 355.8) (GII vs. GI, P=0.04; GII vs GIII, P=0.02), showing contractile recovery in the postconditioning group 10/10s (Table 5 and Figure 2).

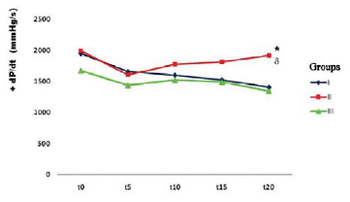
Fig. 2 - Group I, patients with ischemia (control group), Group II, postconditioning group 10/10s, Group III, postconditioning group 30/30s; t0, time immediately before the period of ischemia and t5, t10 , t15, t20, corresponding to 5, 10, 15 and 20min of reperfusion, respectively; mmHg/s, millimeters of mercury per second, * P = 0.02 GII when compared to GIII; P = 0.04 GII when compared to GI.
The restoration of blood flow to the ischemic myocardium is a prerequisite for the interruption of irreversible injury, however, the manner of its application has crucial importance. While the gradual reperfusion can attenuate the deleterious effects after ischemia, abrupt reperfusion has been shown not only increase of the incidence of reperfusion arrhythmias [15] and worsening of ventricular dysfunction [16.17], but also favoring the development of irreversible injury [18-21].
In 2003, Zhao et al. [5] demonstrated experimentally in dogs that ischemic postconditioning reduced the myocardial infarction area. Subsequent studies confirmed the presence of the same behavior in other in vivo animal models (rabbits [22], rats [23]) and in vitro (rabbit [24], rats [25], mice [26]).
Kin et al. [23] observed that after 30min of ischemia in the rat heart
in situ, three or six cycles of reperfusion and ischemia - both with equal length of 10s - reduced the infarcted area in equal proportion. However, when these cycles were initiated after 1min of reperfusion, the protective effect was lost. The first seconds of reperfusion seem to be critical for the cardioprotection mechanism of postconditioning.
In the isolated rat heart, the algorithm of ischemia and reperfusion of 30/30s does not decrease the infarcted area [6,7]. Similarly, the use of three cycles of reperfusion and ischemia, both with equal length of 30s in the pig heart in situ has no cardioprotective effect, suggesting that the algorithm is different from the dog heart
in situ, in addition to variations on collateral circulation between the two species.
The main mediators and effectors involved in the mechanisms of ischemic postconditioning are: adenosine, nitric oxide, the channels of K + ATP-dependent, the pro-survival kinase and the mitochondrial permeability transition pore. The biological expression of these mediators seems to depend on the time of reperfusion/ischemia and animal species. Therefore, it has been suggested that: 1) the length of ischemia and reperfusion in the postconditioning protocol is species-dependent, 2) the number of cycles of ischemia and reperfusion seems to be less important than its length [6,7].
In the study presented herein, changes in heart rate, coronary flow, systolic pressure and the maximum rate of left ventricular pressure drop (-dP/dt max) showed no statistical differences between groups at all times studied (P> 0.05). Significant improvement of contractile function was noted after 20 min of reperfusion by evaluating the maximal rate of rise of left ventricular pressure (+ dP/dt max) in group II in relation to GI (P=0.04) and GIII (P=0.02). Therefore, the postconditioning 10/10s (GII) was effective in preserving the contractile function of isolated rat hearts that underwent 20 min of ischemia and subsequent 20 min of reperfusion. However, the postconditioning 30/30s (GIII) did not show any cardioprotective effect in this study.
Data from literature have suggested that ischemic postconditioning is powerful endogenous mechanism of cardioprotection, because it reduces the infracted area [5], endothelial dysfunction [5], adhesion of neutrophils to vascular endothelium [6], formation of reactive oxygen species [27], reperfusion arrhythmias [15.27], myocardial edema [7] and apoptosis [6] and may require major change in the paradigms of myocardial protection.
The fact of postconditioning protective mechanism does not need to be initiated before the ischemic episode offers many interesting opportunities to the cardiovascular surgeon. It can be used in several situations: 1) after cardioplegic arrest, 2) when the preconditioning is difficult or impossible to perform, 3) in combination with preconditioning; 4) during presentation of acute ischemia, 5) protection of allografts for heart transplants.
CONCLUSION
The ischemic postconditioning with three cycles of reperfusion and ischemia, both with the same period of 10s, was able to preserve contractile function in isolated rat hearts undergone 20 min of ischemia and subsequent 20 min of reperfusion.
REFERENCES
1. Pfeffer JM, Pfeffer MA, Fletcher PJ, Braunwald E. Progressive ventricular remodeling in rat with myocardial infarction. Am J Physiol. 1991;260(5 Pt 2):H1406-14. [MedLine]
2. St John Sutton M, Pfeffer MA, Moye L, Plappert T, Rouleau JL, Lamas G, et al. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation. 1997;96(10):3294-9. [MedLine]
3. Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol. 1960;70:68-78. [MedLine]
4. Braunwald E, Kloner RA. Myocardial reperfusion: a double-edged sword? J Clin Invest. 1985;76(5):1713-9. [MedLine]
5. Zhao ZQ, Corvera JS, Halkos ME, Kerendi F, Wang NP, Guyton RA, et al. Inhibition of myocardial injury by ischemic postconditioning during reperfusion: comparison with ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2003;285(2):H579-88. [MedLine]
6. Vinten-Johansen J, Zhao ZQ, Zatta AJ, Kin H, Halkos ME, Kerendi F. Postconditioning: a new link in nature's armor against myocardial ischemia-reperfusion injury. Basic Res Cardiol. 2005;100(4):295-310. [MedLine]
7. Tsang A, Hausenloy DJ, Yellon DM. Myocardial postconditioning: reperfusion injury revisited. Am J Physiol Heart Circ Physiol. 2005;289(1):H2-7. [MedLine]
8. Princípios Éticos na Experimentação Animal. Colégio Brasileiro de Experimentação Animal - COBEA.
9. Krebs HA, Henseleit K. Untersuchunger über die harnstoffbildung im tierkörper. Hoppe Seylers Z Physiol Chem 1932;210:33-66. Apud in: Doring HJ, Dehnert H. Methods in experimental physology and pharmacology. Reprint of the IST. English Edition, 1987.
10. Gomes OM, Gomes ES, Neves HJ, Guimarães MAC, Pitchon M. Modificação da técnica de Langendorff para estudo de coração isolado com utilização de sistema descartável. VIII Congresso da Sociedade Mineira de Cardiologia, Belo Horizonte, 3 a 5 de Julho de 1997.
11. Langendorff O. Untersuchungen am uberlebenden sangerthierherzen. Arch Ges Physol. 1985;61:291. Apud in: Doring HJ, Dehnert H. Methods in experimental physology and pharmacology. Reprint of the IST. English Edition, 1987.
12. Gleason WL, Braunwald E. Studies on the first derivative of the ventricular pressure pulse in man. J Clin Invest. 1962;41:80-91. [MedLine]
13. Weisfeldt ML, Scully HE, Frederiksen J, Rubenstein JJ, Pohost GM, Beierholm E, et al. Hemodynamic determinants of maximum negative dP-dt and periods of diastole. Am J Physiol. 1974;227(3):613-21. [MedLine]
14. Gottschall C. Determinantes do desempenho cardíaco. In: Gottschall C, editor. Função cardíaca: da normalidade à insuficiência. São Paulo: Fundo Editorial BYK;1995. p.61-72.
15. Galagudza M, Kurapeev D, Minasian S, Valen G, Vaage J. Ischemic postconditioning: brief ischemia during reperfusion converts persistent ventricular fibrillation into regular rhythm. Eur J Cardiothorac Surg. 2004;25(6):1006-10. [MedLine]
16. Hori M, Kitakaze M, Sato H, Takashima S, Iwakura K, Inoue M, et al. Staged reperfusion attenuates myocardial stunning in dogs. Role of transient acidosis during early reperfusion. Circulation. 1991;84(5):2135-45. [MedLine]
17. Bopassa JC, Michel P, Gateau-Roesch O, Ovize M, Ferrera R. Low-pressure reperfusion alters mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2005;288(6):H2750-5. [MedLine]
18. Buckberg GD. When is cardiac muscle damaged irreversibly? J Thorac Cardiovasc Surg. 1986;92(3 Pt 2):483-7. [MedLine]
19. Acar C, Partington MT, Buckberg GD. Studies of controlled reperfusion after ischemia. XVIII. Reperfusion conditions: attenuation of the regional ischemic effect by temporary total vented bypass before controlled reperfusion. J Thorac Cardiovasc Surg. 1990;100(5):737-44. [MedLine]
20. Okamoto F, Allen BS, Buckberg GD, Bugyi H, Leaf J. Reperfusion conditions: importance of ensuring gentle versus sudden reperfusion during relief of coronary occlusion. J Thorac Cardiovasc Surg. 1986;92(3 Pt 2):613-20. [MedLine]
21. Peng CF, Murphy ML, Colwell K, Straub KD. Controlled versus hyperemic flow during reperfusion of jeopardized ischemic myocardium. Am Heart J. 1989;117(3):515-22. [MedLine]
22. Yang XM, Proctor JB, Cui L, Krieg T, Downey JM, Cohen MV. Multiple, brief coronary occlusions during early reperfusion protect rabbit hearts by targeting cell signaling pathways. J Am Coll Cardiol. 2004;44(5):1103-10. [MedLine]
23. Kin H, Zhao ZQ, Sun HY, Wang NP, Corvera JS, Halkos ME, et al. Postconditioning attenuates myocardial ischemiareperfusion injury by inhibiting events in the early minutes of reperfusion. Cardiovasc Res. 2004;62(1):74-85. [MedLine]
24. Yang XM, Philipp S, Downey JM, Cohen MV. Postconditioning's protection is not dependent on circulating blood factors or cells but involves adenosine receptors and requires PI3-kinase and guanylyl cyclase activation. Basic Res Cardiol. 2005;100(1):57-63. [MedLine]
25. Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of "modified reperfusion" protects the myocardium by activating the phosphatidylinositol 3kinase-Akt pathway. Circ Res. 2004;95(3):230-2. [MedLine]
26. Heusch G, Büchert A, Feldhaus S, Schulz R. No loss of cardioprotection by postconditioning in connexin 43-deficient mice. Basic Res Cardiol. 2006;101(4):354-6. [MedLine]
27. Halkos ME, Kerendi F, Corvera JS, Wang NP, Kin H, Payne CS, et al. Myocardial protection with postconditioning is not enhanced by ischemic preconditioning. Ann Thorac Surg. 2004;78(3):961-9.
Article receive on Friday, July 4, 2008



 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license