ABSTRACT
OBJECTIVE: To assess the histological changes of the aorta, the renal arteries and the renal parenchyma in swine, induced by a metalic uncovered stent implanted in transrenal position in the abdominal aorta. METHODS: Ten pigs with a mean weight of 86.6 kg and mean age of 6 months underwent implantation of metal stent graft placed in the aorta at the level of the renal arteries after 100 days of implantation. The self-expanding stents were released by laparotomy. Anatomic and histological analyses of the abdominal aorta, the renal arteries and the renal parenchyma were performed. Histological slices were performed in the following sites: 1) transitional zone between the aorta with and without stent graft; 2) portion of the renal arteries ostia; 3) renal parenchyma. The slices were stained through the hematoxylin and eosin stain technique and analyzed according the protocol of histological analyses applied in the clinical practice of pathology labs. RESULTS: The macroscopic findings showed thickening of the aortic wall; patent renal arteries; and normal anatomic renal structures. Microscopic analyses, close to the stents, showed thickening of the vascular wall, renal arteries without changes, and preserved renal parenchyma. CONCLUSION: The uncovered stainless steel stent caused a significant inflammatory reaction with thickening of the aortic wall. However, the renal arteries remained patent and the renal parenchyma did not present embolic or ischemic changes.
RESUMO
OBJETIVO: Avaliar as alterações histológicas da aorta, artéria renal e parênquima renal, em suínos, induzidos pelo stent metálico descoberto implantado em localização transrenal na aorta abdominal. MÉTODOS: Foram utilizados 10 suínos com peso médio de 86,6 quilos e idade média de 6 meses, submetidos a implante de stent metálico posicionado na aorta, no nível das artérias renais, após 100 dias do implante. Os stents foram liberados por auto-expansão com laparotomia. Foram realizadas análises anatômicas e histológicas da aorta abdominal, artérias renais e parênquima renal. Os cortes histológicos foram realizados nos seguintes locais: 1) transição entre a aorta normal e aorta contendo stent; 2) porção contendo os óstios das artérias renais, 3) parênquima renal. As lâminas foram coradas pela técnica da hematoxilina e eosina e analisadas conforme protocolo de análise histológica aplicada na prática clínica dos laboratórios de patologia. RESULTADOS: Os achados macroscópicos revelaram espessamento da parede aórtica; artérias renais pérvias; estrutura anatômica renal normal. Análises microscópicas, próximas aos stents, evidenciaram espessamento da parede vascular, artérias renais sem alterações e parênquima renal preservado. CONCLUSÃO: O stent de aço inoxidável descoberto produziu importante reação inflamatória com espessamento da parede da aorta. No entanto, as artérias renais permaneceram pérvias e o parênquima renal sem alterações isquêmicas ou embólicas.
INTRODUCTION
The endovascular treatment has been widely used in the treatment of abdominal aortic aneurysm (AAA) and has been shown to be an important alternative to conventional surgical repair, especially in patients with associated comorbidities [1-3].
The technique of fixation on the ostium of the stent's renal or adrenal arteries was originally proposed by Lawrence et al. [4] in order to prevent the migration and optimize the hemostatic pole in the cephalic extremity of the stent. Soon after, the application of this technique has spread also to the implantation of infrarenal stent with satisfactory results [5-9].
The endovascular repair of aortic aneurysm is an alternative therapy performed through the implantation of endoprosthesis by transvascular route, on which the main purpose is to exclude the aneurysm from the systemic circulation. The insertion of an aortic endoprosthesis causes an endothelial inflammatory reaction producing macroscopic, microscopic and laboratory changes [10-13].
Although most of the publications available on intravascular procedures include studies on coronary arteries, the mechanisms of formation of neointima in other human arteries - such as iliac arteries and aorta - are considered similar [14]. Studies have shown signs of inflammatory reaction after stent placement in both aorta and other arteries and its relation to NIH and its complications, specially restenosis, in humans and animal models [13].
Among the changes produced after implantation of aortic stents, the renal effects have been increasingly approached by the literature. The physiological effects on renal function after fixation of non-covered metallic stent on the ostium of the renal arteries are not completely known. Several studies report that the fixation to the renal artery has been shown to be safe in short- and mid-term assessments [15-24]. These studies have shown through biochemical and imaginological analysis that the implantation of adrenal stents is safe. However, the long-term histological effects of this technique are still not fully understood [8, 25-27]. Among the consequences of adrenal stents implantation we can highlight changes in renal flow, renal artery ostium stenosis and changes in the aortic wall in response to the stent [28,29]. We showed in a recent study the histological changes induced by non-covered stent produced in the aortic endothelium. We noted a significant neointimal hyperplasia limited to the region of the stent [13].
In this study, we will approach the patency and histological reactions in the ostium of the renal arteries, as well as changes in renal parenchyma induced by non-covered steel stent placed in adrenal topography in the abdominal aorta of pigs. Histological analyses were performed by a pathologist following protocols for histopathological analysis applied in clinical practice.
METHODS
The experiments were performed in the Veterinary Hospital of the University of the State of Santa Catarina in Lages, SC. The project was approved in the Animal Experimentation Ethics Committee of the Paulista Medical School - UNIFESP under No. 0845/03 on 07/25/2003.
We used 10 pigs from F1 Landrace x Large White cross-combination from pig farming of the Veterinary Agro Center (CAV) with mean weight of 86.6 kg (± 2.44) and mean age of 6.35 months (± 0.15) in a good sanitary condition. The animals underwent 12 hours of fasting prior to surgical procedures.
All animals were pre-medicated with a combination of atropine 0.025 mg/kg/IM and with an interval of 10 minutes was administered xylazine 1 mg/kg - all via IM route. After sedation and using a catheter, venoclysis of the marginal ear vein was performed for administration of anesthetic drugs and fluid therapy (5mL/kg/hour). Another catheter was placed in the central ear artery to measure blood pressure directly and blood collection for gasometry. After induction, lidocaine 10% spray was used to desensitization of vocal cords and larynx, followed by intubation with endotracheal tube using a low-pressure balloon in accordance with the tracheal diameter. After appropriate intubation and inhalation anesthesia with halothane, 1.5 MAC (minimum alveolar concentration) was maintained in a semi-closed circuit with oxygen flow of 40ml/kg. Then the animals were placed on mechanical ventilation using a volume ventilator (15 ml per kilogram body weight). During the anesthetic procedure were assessed the following parameters: heart rate, respiratory rate, systolic, diastolic and mean blood pressure measurements, through noninvasive monitoring and esophageal temperature.
With trans-mesenteric dissection maneuvers abdominal aorta was identified in the junction of the renal arteries and a purse with 4-0 propylene yarn was performed, 5 cm below the renal arteries that was introduced by puncturing of an "extra-hard" guidewire with diameter of 0.032mm or 0.035mm. A non-covered self-expandable aortic stent supplied commercially by Braile BiomédicaÒ and inserted inside a PTFE catheter, with diameters of 16, 18 and 20 mm according to the aortic diameter measured by a caliper before introduction. After introduction of an "extra hard" metal guidewire the aortic abdominal aortic stent was released by positioning it in front of the renal arteries through measurements in the pre-introduction that are taken in the system by marking in its structure using a long silk thread or the own guidewire and identified by digital maneuvers. Using a conventional ultrasonic apparatus it was confirmed the position of the aortic stent, with placement of the transducer directly on the aorta on which the stent was released.
After placing of the stent, the drilling peformed by puncturing was closed by the purse previously performed with the 4-0 propylene yarn without placement of vascular clamp and without administration of any systemic anticogulant. Also, layer closure of the abdominal cavity was performed and without placement of drains. In the postoperative period was used a combination of penicillin-based antibiotics and streptomycin every 24 hours associated with the use of an analgesic-based ketoprofen at a dose of 0.1 mg/kg which were maintained for 3 days. With routine care with food and hygiene the animals were maintained for 100 days awaiting the slaughter in commercial refrigerator supervised by the health surveillance.
After slaughter, the animals had their abdominal aorta, renal arteries and kidneys dissected and removed for anatomical and histological analysis. The macroscopic examination of kidneys and aorta was performed immediately after removal. Then, the material was packed in thermal bag with ice and sent for histological analysis. Histological analyses were performed by a pathologist using methods well-established and applied in clinical practice of pathology laboratories.
RESULTS
The results found repeated in all samples. Therefore, the results are presented descriptivelly and illustrated by figures representing these changes found in all samples.
Macroscopy
In figure 1 is shown the aortic segment, on which the stent was positioned, with longitudinal cutting. We can observe the transition between the normal aortic wall and the wall around the stent. We show the structure of the stent completely covered by a thin layer of tissue with thickening in the regions of the stent's metal supports.
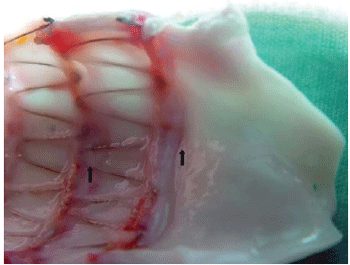
Fig. 1 - Longitudinal aortic cut, on which we can see the transition between the normal aortic wall and the region thickened by reactions induced by the stent
It is shown in Figure 2, the structure of the stent covered with a thin layer of tissue, but the renal arteries remained patent without signs of obstruction.
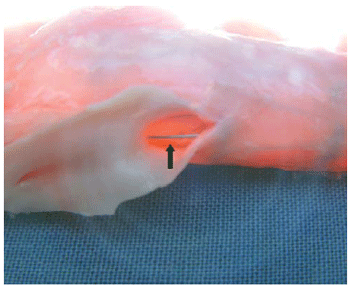
Fig. 2 - External view of the abdominal aorta with a metallic support of the stent (arrow) passing on the ostium of the renal artery without signs of obstruction
Microscopic examination has shown preserved kidney structures, without embolic signs or ischemic changes in all animals studied (Figure 3).
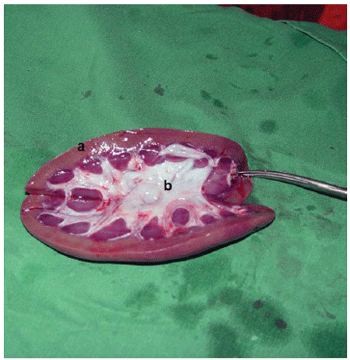
Fig. 3 - Longitudinal cut of the kidney. a - Cortex. b - Marrow
Figure 4 shows the longitudinal cut of the aorta, stained with HE, in the transition between the normal distal segment, the gradual thickening of the intimal layer, as it approaches the stent, showing the inflammatory reaction induced by the stainless steel stent.
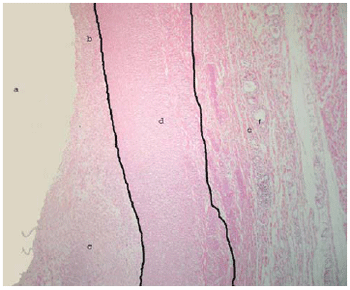
Fig. 4 - Longitudinal cut in the transition between the normal distal segment and the gradual thickening of the aorta: (a) aortic lumen, (b) intimal layer with normal thickness, (c) thickened intimal layer (extremity closest to the stent) with accumulation of fibroblasts and collagen, (d) middle layer (muscular) containing smooth muscle cells, (e) adventitia layer containing fat cells, loose connective tissue and vasa vasorum, (f) vasa vasorum. HE 100X
Figure 5 shows the normal appearance of the renal artery in the region near the stent.
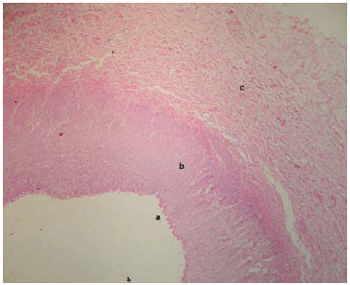
Fig. 5 - Cross-section of the renal artery in front of the stent. (a) intimal layer, (b) middle muscular layer, (c) adventitia layer. HE 100X
We can see in Figure 6, the histology of the normal renal parenchyma without signs of embolic or ischemic events.
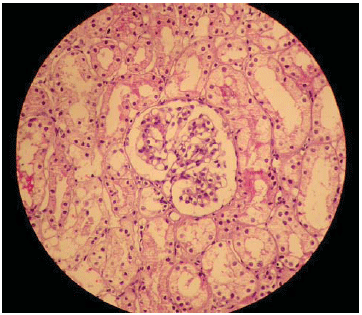
Fig. 6 - Renal histological cut showing normal structures
The histological and physiological effects of fixing of the transrenal aortic stent are not yet fully known. Experimental studies have shown that the arterial wall shows a multifactorial response to mechanical lesion called intimal hyperplasia. Topographically, this response occurs primarily in the tunica intima and is characterized by cell proliferation and intimal thickening that can result in significant reduction of the vessel lumen [30, 31-33]. Our group, in a previous experimental study showed significant inflammatory reaction in the aortic wall limited to the region of the stent without compromising the aortic patency [13].
Several studies monitored renal function by dosing biochemical markers such as serum urea, serum creatinine and glomerular filtration rate after implantation of andrenal aortic stent [7,8,23]. These studies showed no short- and mid-term significant changes in renal function after the procedure.
Sun and Stevenson performed an extensive short- and mid-term systematic review by assessing renal function after fixation of the adrenal stent. The authors concluded that the fixation of non-covered stents on the ostia of the renal arteries produced no significant renal dysfunction in the studies reviewed [33].
We evaluated the histological reaction produced by the stainless steel stent and reinforced with polyester yarn in the aorta of our animals. We noticed that there was a significant inflammatory reaction in the region on which the stent was placed (Figure 4). Around the cavity produced by the metal wire, we noted a pronounced thickening of the intimal layer (Figure 6). With these findings we can state that there was significant neointimal hyperplasia secondary to an intense inflammatory response.
We also clearly verified that the structural changes in the aortic wall were limited to the region on which the stent was placed (Figure 2). Macroscopically we observed patency of the renal arteries (Figure 3) transfixed by the stent's metal wire. Additionally, histological analysis of the renal arteries did not reveal any abnormality, with no signs of occlusion. These evidences show that the endothelial reaction induced by the non-covered aortic metallic stent did not compromise the renal circulation.
The anatomical analysis of the renal structure showed normal organs, with no signs of ischemic or embolic compromising (Figure 3). The histological analysis confirmed in details the absence of changes in glomerular level (Figure 6) reinforcing the evidence that the stent did not produce lesion in the kidney.
Our findings clearly show that the non-covered stainless steel stent implanted in the aorta of pigs produced a significant inflammatory reaction evidenced by macroscopical analysis and confirmed by histology. However, it was evident in this study that the fixation of the stent in front of the renal artery did not compromise the patency of the renal arteries and did not produce histological damage in the renal parenchyma.
REFERENCES
1. Buth J, van Marrewijk CJ, Harris PL, Hop WC, Riambau V, Laheij RJ, et al. Outcome of endovascular abdominal aortic aneurysm repair in patients with conditions considered unfit for an open procedure: a report on the EUROSTAR experience. J Vasc Surg. 2002;35(2):211-21. [MedLine]
2. Cao P, Verzini F, Parlani G, Romano L, De Rango P, Pagliuca V, et al. Clinical effect of abdominal aortic aneurysm endografting: 7-year concurrent comparison with open repair. J Vasc Surg. 2004;40(5):841-8. [MedLine]
3. Prinssen M, Verhoeven EL, Buth J, Cuypers PW, van Sambeek MR, Balm R, et al. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004;351(16):1607-18. [MedLine]
4. Lawrence DD Jr, Charnsangavej C, Wright KC, Gianturco C, Wallace S. Percutaneous endovascular graft: experimental evaluation. Radiology. 1987;163(2):357-60. [MedLine]
5. Kichikawa K, Uchida H, Maeda M, Ide K, Kubota Y, Sakaguchi S, et al. Aortic stent-grafting with transrenal fixation: use of newly designed spiral Z-stent endograft. J Endovasc Ther. 2000;7(3):184-91. [MedLine]
6. Lobato AC, Quick RC, Vaughn PL, Rodriguez-Lopez J, Douglas M, Diethrich EB. Transrenal fixation of aortic endografts: intermediate follow-up of a single-center experience. J Endovasc Ther. 2000;7(4):273-8. [MedLine]
7. Izzedine H, Koskas F, Cluzel P, Mallet A, Maksud P, Deray G. Renal function after aortic stent-grafting including coverage of renal arterial ostia. Am J Kidney Dis. 2002;39(4):730-6. [MedLine]
8. Grego F, Frigatti P, Antonello M, Lepidi S, Ragazzi R, Iurilli V, et al. Suprarenal fixation of endograft in abdominal aortic aneurysm treatment: focus on renal function. Ann Surg. 2004;240(1):169-78. [MedLine]
9. Greenberg RK, Chuter TA, Lawrence-Brown M, Haulon S, Nolte L; Zenith Investigators. Analysis of renal function after aneurysm repair with a device using suprarenal fixation (Zenith AAA Endovascular Graft) in contrast to open surgical repair. J Vasc Surg. 2004;39(6):1219-28. [MedLine]
10. Gomes WJ, Buffolo E. Coronary stenting and inflammation: implications for further surgical and medical treatment. Ann Thorac Surg. 2006;81(5):1918-25. [MedLine]
11. Gabriel EA, Locali RF, Romano CC, Duarte AJ, Palma JH, Buffolo E. Analysis of the inflammatory response in endovascular treatment of aortic aneurysms. Eur J Cardiothorac Surg. 2007;31(3):406-12. [MedLine]
12. Schneider DB. Endovascular treatment of descending thoracic aortic aneurysms. In: Mastery of vascular and endovascular surgery. 1st ed. Philadelphia:Lippincott Williams & Wilkins;2006. p.85-93.
13. Bombonato R, Palma JH, Marcondes JA, Moraes AN, Martins MR, Tchaick RM, et al. Reação histopatológica da parede da aorta abdominal ao stent não recoberto. Rev Bras Cir Cardiovasc. 2006;21(2):198-205. View article
14. Byer A, Ussia G, Galleti G. Autologous vein lined and vein covered stents in swine arteries. An experimental study to assess and compare patency and intimal hyperplastic response. J Cardiovasc Surg. 1998;39(4):393-8.
15. Lawrence-Brown MM, Hartley D, MacSweeney ST, Kelsey P, Ives FJ, Holden A, et al. The Perth endoluminal bifurcated graft system: development and early experience. Cardiovasc Surg. 1996;4(6):706-12. [MedLine]
16. Shames M, Betros F, Dennien B, Gray-Weale A, Lippey E, Thursby P, et al. Transrenal versus infrarenal endograft fixation: influence on type I endoleaks. Ann Vasc Surg. 2002;16(5):556-61. [MedLine]
17. Carpenter JP, Fairman RM, Barker CF, Golden MA, Velazquez OC, Mitchell ME, et al. Endovascular AAA repair in patients with renal insufficiency: strategies for reducing adverse renal events. Cardiovasc Surg. 2001;9(6):559-64. [MedLine]
18. Greenberg RK, Lawrence-Brown M, Bhandari G, Hartley D, Stelter W, Umscheid T, et al. An update of the Zenith endovascular graft for abdominal aortic aneurysms: initial implantation and mid-term follow-up data. J Vasc Surg. 2001;33(2 Suppl):S157-64. [MedLine]
19. Böckler D, Krauss M, Mansmann U, Halawa M, Lange R, Probst T, et al. Incidence of renal infarctions after endovascular AAA repair: relationships to infrarenal versus suprarenal fixation. J Endovasc Ther. 2003;10(6):1054-60. [MedLine]
20. Bove PG, Long GW, Shanley CJ, Brown OW, Rimar SD, Hans SS, et al. Transrenal fixation of endovascular stent-grafts for infrarenal aortic aneurysm repair: mid-term results. J Vasc Surg. 2003;37(5):938-42. [MedLine]
21. Greenberg RK, Chuter TA, Sternbergh WC 3rd, Fearnot NE; Zenith Investigators. Zenith AAA endovascular graft: intermediate-term results of the US multicenter trial. J Vasc Surg. 2004;39(6):1209-18. [MedLine]
22. Hinchliffe RJ, Goldberg J, Macsweeney ST; Zenith Users Group. A UK multi-centre experience with a second-generation endovascular stent-graft: results from the Zenith Users Group. Eur J Vasc Endovasc Surg. 2004;27(1):51-5. [MedLine]
23. Mehta M, Cayne N, Veith FJ, Darling RC 3rd, Roddy SP, Paty PS, et al. Relationship of proximal fixation of renal dysfunction in patients undergoing endovascular aneurysm repair. J Cardiovasc Surg (Torino). 2004;45(4):367-74. [MedLine]
24. Surowiec SM, Davies MG, Fegley AJ, Tanski WJ, Pamoukian VN, Sternbach Y, et al. Relationship of proximal fixation to postoperative renal dysfunction in patients with normal serum creatinine concentration. J Vasc Surg. 2004;39(4):804-10. [MedLine]
25. Malina M, Lindh M, Ivancev K, Frennby B, Lindblad B, Brunkwall J. The effect of endovascular aortic stents placed across the renal arteries. Eur J Vasc Endovasc Surg. 1997;13(2):207-13. [MedLine]
26. Espinosa G, Marchiori E, Silva LF, Araújo AP, Riguetti C, Baquero RA. Initial results of endovascular repair of abdominal aortic aneurysm with a self-expanding stent-graft. J Vasc Interv Radiol. 2002;13(11):1115-23. [MedLine]
27. Cayne NS, Rhee SJ, Veith FJ, Lipsitz EC, Ohki T, Gargiulo NJ 3rd, et al. Does transrenal fixation of aortic endografts impair renal function? J Vasc Surg. 2003;38(4):639-44. [MedLine]
28. Desgranges P, Hutin E, Kedzia C, Allaire E, Becquemin JP. Aortic stents covering the renal arteries ostia: an animal study. J Vasc Interv Radiol. 1997;8(1 Pt 1):77-82. [MedLine]
29. Sun Z, Zheng H. Cross-sectional area reduction of the aortic ostium by suprarenal stent wires: in vitro phantom study by CT virtual angioscopy. Comput Med Imaging Graph. 2004;28(6):345-51. [MedLine]
30. Schwartz RS. Pathophysiology of restenosis: interaction of thrombosis, hyperplasia, and/or remodeling. Am J Cardiol. 1998;81(7A):14E-17E.
31. Castagna MT, Mintz GS, Leiboff BO, Ahmed JM, Mehran R, Satler LF, et al. The contribution of "mechanical" problems to in-stent restenosis: an intravascular ultrasonographic analysis of 1090 consecutive in-stent restenosis lesions. Am Heart J. 2001;142(6):970-4. [MedLine]
32. Sullivan TM, Ainsworth SD, Langan EM, Taylor S, Snyder B, Cull D, et al. Effect of endovascular stent strut geometry on vascular injury, myointimal hyperplasia, and restenosis. J Vasc Surg. 2002;36(1):143-9. [MedLine]
33. Sun Z, Stevenson G. Transrenal fixation of aortic stent-grafts: short-to midterm effects on renal function--a systematic review. Radiology. 2006;240(1):65-72. [MedLine]
Article receive on Wednesday, September 17, 2008






 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license