ABSTRACT
OBJECTIVE: Infectious and inflammatory processes mediated by bacteria in distant sites have been described as a risk factor for acute ischemic heart disease (AIHD). METHODS: One hundred one patients with AIHD with and without chronic periodontitis (CP) were included in this study. Patients were admitted to the HC UNICAMP and stratified into three groups: in group 1, we selected patients with severe chronic periodontitis (31 men and 19 women, mean age 55.1 ± 11.29 years old); the group 2 with mild chronic periodontitis (40 men and 28 women, mean age 54.8 ± 10.37 years old) and group 3 represented by the toothless (43 men and 20 women, mean age 67.5 ± 8.55 years old). Blood samples were collected to measure the lipid profiles, hematological and blood glucose levels. In addition, biopsies of seventeen coronary arteries with atherosclerosis and an equal number of internal mammary arteries without atherosclerotic degeneration in group 1 were investigated. Statistical analysis by analysis of variance (ANOVA) and Scheffé test for multiple comparisons was performed. RESULTS: Triglyceride and LDL levels were elevated in group 1 than in group 2. HDL were reduced by 20% in group 1 and remained reduced by 8% in toothless. Blood glucose was higher in group 1. DNA of periodontal bacteria was detected in 58.8% of the coronary arteries. CONCLUSIONS: Patients with (AIHD) and severe chronic periodontitis may have altered lipid profile, as well as microorganisms associated with CP can permeate into coronary vessels.
RESUMO
OBJETIVO: Processos inflamatórios e infecciosos mediados por bactérias em sítios distantes têm sido descritos como fator de risco à doença coronariana isquêmica aguda (DCIA). MÉTODOS: Cento e oitenta e um pacientes com DCIA, com e sem periodontites crônicas, foram incluídos neste estudo. Os pacientes foram admitidos no HC da UNICAMP e estratificados em três grupos: grupo 1 - pacientes com periodontite crônica grave (31 homens e 19 mulheres; média de idade 55,1 ± 11,29 anos); grupo 2 - pacientes com periodontite crônica leve (40 homens e 28 mulheres; média de idade 54,8 ± 10,37 anos); grupo 3 - pacientes desdentados (43 homens e 20 mulheres; média de idade 67,5 ± 8,55 anos). Amostras sanguíneas foram coletadas para mensurar os perfis lipídico, hematológico e glicêmico. Além disso, biópsias de 17 artérias coronárias com aterosclerose e igual número de artérias mamárias internas sem degeneração aterosclerótica no grupo 1 foram investigadas. Para análise estatística utilizou-se a análise de variância (ANOVA) e o teste de Scheffé para comparações múltiplas. RESULTADOS: Triglicérides e LDL estavam elevados no grupo 1 em relação ao grupo 2. O HDL apresentou-se reduzido em 20% dos pacientes do grupo 1, e em 8% nos desdentados. A glicemia estava elevada no grupo 1. DNA de bactérias periodontais foram detectados em 58,8% das artérias coronárias. CONCLUSÕES: Pacientes com DCIA e periodontite crônica grave podem apresentar perfil lipídico alterado, como também microorganismos associados com as periodontites crônicas graves podem permear dentro de vasos coronarianos.
INTRODUCTION
The chronic periodontitis presents as chronic infectious diseases characterized by inflammatory changes in periodontal tissues. These are mainly caused by gram-negative bacteria, including
Porphyromonas gingivalis (Pg),
Prevotella intermedia (Pi),
Aggregatibacter actinomycetemcomitans (Aa) and
Tannerella forsythia (Tf)[1].
Chronic bacterial infections caused by Chlamydia pneumoniae and chronic periodontitis, as well as certain viruses have been associated with risk for systemic conditions such as coronary artery disease and atherosclerosis [2]. Species associated with chronic periodontitis can enter the blood flow through gingival blood vessels and migrate to the atheromatous plaques [3].
Traditional risk factors and well-established coronary diseases, including hypertension, diabetes, obesity, smoking and physical inactivity does not fill half of the cases affected with this disease [4].
Hypercholesterolemia, specially high levels of low density lipoproteins (LDL-C), hypertriglyceridemia and low levels of high density lipoprotein (HDL-C) are major risk factors for acute coronary syndromes [5].
The basis of this study was to observe patients presenting with acute ischemic heart disease with and without chronic periodontitis, the behavior of the lipid, hematological and blood biochemical profile as well as assessing biopsies of internal mammary and coronary arteries to detect the presence of Chlamydia pneumoniae and bacteria related to
chronic periodontitis.
METHODS
Sample
One hundred and eighty-one patients with acute ischemic heart disease, diagnosed by electrocardiography and/or angiography as well as clinically and biochemically (CK,CK) are included in this study. All patients were informed verbally and in writing to participate in the research, and the study was approved by the Research Ethics Committee of the Faculty of Medical Sciences at UNICAMP. The periodontal examinations were performed within the first 24 hours, from admission at coronary care unit of HC/UNICAMP. Of the total patients, 63 are toothless as gender distribution, mean age and standard deviation (43 men and 20 women, mean age 67.5±8.55 years). The remaining 118 patients were subdivided into two subgroups: fifty presented severe chronic periodontitis (31 men and 19 women, mean age 55.1±11.29 years), with more than 30% of teeth affected with loss of periodontal clinical attachment level > 4 mm, with more than five periodontal pockets with probing depth > 5 mm and presenting at least 20 dental units) and the other with mild chronic periodontitis (40 men and 28 women, mean age 54.8±10.37 years), with less than 30% of teeth affected with loss of clinical attachment level < 3 mm and without periodontal pockets, as well as 20 dental units. This classification of mild and severe chronic periodontitis is accoding with consensus on chronic periodontitis of the American Academy of Periodontology [6].
Samples of 17 coronary arteries with atherosclerosis and same number of internal mammary artery (used as grafts) with no atherosclerotic degeneration were investigated for detection of periodontal pathogens and Chlamydia pneumoniae. Fragments of specimens of coronary and mammary artery were obtained during surgery for coronary artery bypass grafting, and were removed from the site of the anastomosis in the coronary arteries and distal segments of mammary grafts during its preparation. These patients were followed-up by the Cardiac Surgery and Cardiology Discipline Department at Clinics Hospital of the Faculty of Medical Sciences, State University of Campinas (HC-UNICAMP). All arterial specimens were obtained from the same group of patients with severe chronic periodontitis.
Immediately after the biopsy, the arterial specimens were frozen in nitrogen and stored at -80°C. The detection of pathogens was performed by analysis of polymerase chain reaction (PCR).
PCR and DNA extraction
Vessels removed were treated with proteinase K at 56ºC for 30 minutes followed by 10 minutes of proteinase K inactivated at 95ºC, and with 5 minutes centrifugation in a microcentrifuge to remove cellular debris. PCR was performed in volumes of 25µl containing PCR/Mg++ buffer, 0.2 µM of deoxycytosine triphosphate (dCTP), deoxyguanosine triphosphate (dGTP), deoxyadenosine triphosphate (dATP), and deoxythymidine triphosphate (dTTP), 0.2µM of each primer, 0.5 U Taq DNA polymerase, and 3-5 µl template DNA containing supernatants. The amplification was performed in a DNA thermal cycler programmed to 94ºC (5 minutes), followed by 35 cycles at 94ºC (30 seconds), annealing temperature appropriate for each primer pair (30 seconds) and extension at 72ºC (1 minute and 30 seconds), plus a final extension at 72ºC (5 minutes). The PCR amplified fragments were visualized on polyacrylamide gel at 8%, stained with ethidium bromide on UV transilluminator. The primers used in the study include: Universal primer Escherichia coli 16S rDNA (pF1: 5 AGA GTT TGA TCC TGG CTCAG 3)(E. coli - position: 28-27), Aa (Aa1: 5 CAC TTA AAG GTC CGC CTA CGT GC 3) C. pneumonia (HL-1-GTT GTT CAT GAA GGC CCT ACT HR-1-TGC ATA ACC TAC GGT TGT GTT) Pg (Pg1: 5 CAA TAC TCG TAT CGC CCG TTA TTC 3) Pi (Pi: 5 GTT GCG TGC ACT CAA GTC CGC C 3), Tf (TF V530: 5 GTA GAG CTT ACA CTA TAT CGC AAA CTC CTA 3).
American Type Culture Collection (ATCC) cultures of Pg (ATCC 33277), Aa (ATCC 33 384), Tf (ATCC 43 037) and Pi (ATCC 25 611) were used as positive control. The negative control was a PCR mix without DNA.
Medical History
Demographic features, individual habits and cause of loss of teeth units in toothless were also observed. Patients in the study showed no other cardiac or infectious disease, and were not taking any medication or antibiotic drugs that could reduce cholesterol levels, as well as not having undergone any periodontal treatment in the last six months.
Laboratory analysis
When admitted to the coronary care unit, all patients in the study underwent fasting blood sample for diagnosis of lipid, glycemic and haematological profile. All examinations were performed in the clinical pathology laboratory of the hospital.
The following limit values for lipids and lipoproteins, according to the National Cholesterol Education Program - Adult Treatment Panel III (NCEP-ATP III) [11] were considered: total cholesterol (TC): desirable < 200mg/dL, borderline risk 200-239mg/dl and high 240mg/dl. Triglycerides (TG): normal < 150mg/dl borderline 150-199mg/dl, high 200-499mg/dl and very high 500mg/dl. HDL: low < 40mg/dl; high 60mg/dl. LDL: optimal < 100mg/dl and above optimal 100-129mg/dl; limit of the high 130-159mg/dl, high 160-189mg/dl and very high 190mg/dl.
Fasting glucose and white cell count had the limits: ≤ 110 mg/dl and 4000-10000mm
3, respectively.
Periodontal Examinations
The patients were periodontally examined by a single examiner, using a mouth mirror, direct lighting and manual probe PCP-UNC 15 in the hospital bed. The PCP-UNC 15 probe was used to detect the level of clinical attachment and periodontal pocket depth and is expressed as the distance in millimeters from the cemento-enamel junction in the dental unit and gingival margin at the bottom of the gingival pocket. These parameters were measured at six sites per tooth (mesiobuccal, mid-buccal, disto-vestibular, mesiolingual, mid-lingual and distal-lingual) in the contralateral superior and inferior quadrants, with a threshold of loss of clinical attachment level greater than 4 mm and > 5mm in the periodontal pockets, being considered affected by severe chronic periodontitis and also presenting at least ≥ 20 dental units.
The patients' age and the variables that compose the lipid profile, as well as white cell counts were described by mean and standard deviation.
Statistical analysis
The hypothesis of equality between these means in the three groups was tested by analysis of variance (ANOVA). Comparisons between each possible pair of means were assessed using the Scheffé test [7], considering statistically significant the differences between means whose P value was less than 5%. All these data were assessed using the software STAT-VIEW 5.0 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The study was performed from November 2000 to May 2005 when we selected 181 patients with acute ischemic coronary disease, by chronological addition at HC/ UNICAMP, according to the inclusion criteria specified.
Figures 1-3 show the groups with and without chronic periodontitis and traditional risk factors of acute ischemic heart disease.
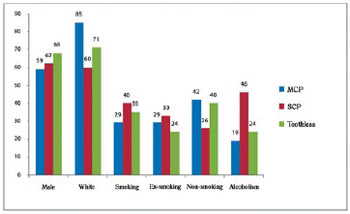
Fig.1 - Demographic characteristics of patients with coronary artery disease with and without chronic periodontitis. SCP = severe chronic periodontitis; MCP = mild chronic periodontitis
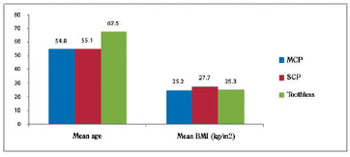
Fig. 2 - Demographic characteristics of patients with coronary artery disease with and without chronic periodontitis. BMI = Body mass index; SCP = severe chronic periodontitis; MCP = mild chronic periodontitis
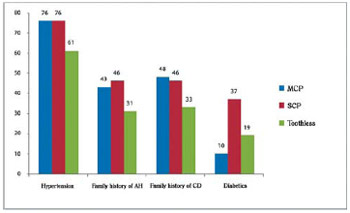
Fig. 3 - Clinical parameters of patients with coronary artery disease with and without chronic periodontitis. AH = arterial hypertension; CHD = Coronary heart disease; SCP = severe chronic periodontitis; MCP = mild chronic periodontitis
Age is a factor all with high prevalence in the incidence of tooth loss, with statistically significant differences between groups with chronic periodontitis and toothless, but no statistical significance between those with mild and severe chronic periodontitis.
The body mass index was equivalent in all three groups, according to the classification of obesity in adults from the World Health Organization [8].
It was observed prevalence of male gender, white race and hypertension, on which these percentages were above 70% on average in the three groups.
Family history of hypertension and cardiovascular diseases have not reached the percentage of 50% on average in the three groups.
The incidence of diabetes in both groups was around 20% with higher prevalence in the group with severe chronic periodontitis in approximate percentage of 37%.
Figure 4 shows the means and standard deviations in groups of lipid and glycemic profiles. Although no statistically significant differences between groups in lipoproteins (HDL-C, LDL-c) were found, we observed reduced values of the limit of HDL-cholesterol that could be considered low, with a drop more pronounced in the group with severe chronic periodontitis in approximately 20% as well as 8% in the toothless group.
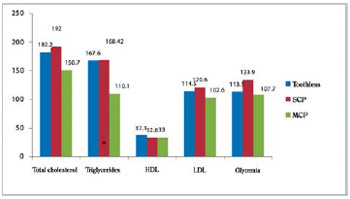
Fig. 4 - Lipid and glycemic profile in patients with coronary artery disease with and without mild and severe chronic periodontitis. HDL = High-density lipoprotein, LDL = Low density lipoprotein; SCP = severe chronic periodontitis; MCP = mild chronic periodontitis. Comparison of means through analysis of variance (ANOVA), adjusted by age. Statistically significant difference (P.0.05) between MCP and toothless. Difference statistically significant (P. 0.05) between SCP and MCP
Moreover, LDL-cholesterol was above the normal range and therefore away from the value considered optimal, in approximately 20% in patients with severe chronic periodontitis and 15% in toothless patients, but with normal values in the group with mild chronic periodontitis.
Triglycerides and total cholesterol were high in about 53% and 27% respectively in the group with severe chronic periodontitis in relation to the group with mild chronic periodontitis. However, these lipids (triglycerides and total cholesterol) were similar between the groups with severe chronic periodontitis and the toothless group.
Triglycerides and LDL-cholesterol levels were approximately 13% and 20% above the limit values in the groups with severe chronic periodontitis and toothless group, while total cholesterol was in normal levels in the three groups.
Blood glucose was high in approximately 20% of the limit in the group with severe chronic periodontitis, but with normal values in other groups.
The white cell count was more high in the group with severe chronic periodontitis in approximately 43%, while in groups with mild chronic periodontitis and toothless were high in 20% (Figure 5).
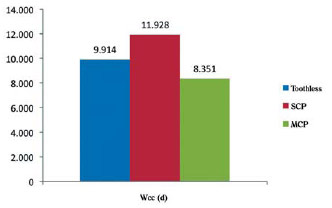
Fig. 5 - Hematological profile of patients with coronary artery disease with and without mild and severe chronic periodontitis. Wcc = white cell count; SCP = severe chronic periodontitis, MCP = mild chronic periodontitis; Wcc was converted into a logarithmic scale for comparison of means
DNA from periodontal bacteria were detected in samples from the coronary artery in the following proportions: Porphyromonas gingivalis (52.9%), Aggregatibacter actinomycetemcomitans in six (35.3%) biopsies, Prevotella intermedia in four (23.5%), and Tannerella forsythia in two (11.7%) samples. Seven (41.1%) specimens were positive for two or more periodontal bacteria. The association between Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans was found in ten of the 17 arteries studied (Figure 6).
Periodontal pathogens were not detected in internal mammary arteries. Chlamydia pneumonia was detected in six (35.3%) internal mammary arteries and coronary. Mixed infection with Chlamydia and periodontal pathogen was observed in four specimens of the coronary artery (Figure 6).
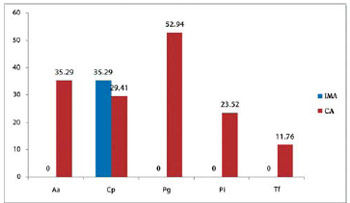
Fig. 6 - Analysis of arterial samples infected by microorganisms (SCP group). SCP Group = severe chronic periodontitis; IMA = internal mammary artery; CA = coronary artery; Aa = Aggregatibacter actinomycetemcomitans; Cp = Chlamydia pneumoniae; Pg = Porphyromonas gingivalis, Pi = Prevotella intermedia, Tf = Tannerella forsythia
Several studies have shown an association between chronic periodontitis and levels of serum lipoproteins, but with some controversy between them. Studies suggest that there is a relationship between levels of total cholesterol and periodontitis [9,10], while other researchers have found a relationship between triglyceride levels and periodontitis [11,12]. However, another group of researchers found that chronic periodontitis were associated with high levels of total cholesterol and triglycerides [13], demonstrating a similar relationship with our research.
In our study total cholesterol level was normal in all three groups, but was higher in the group with severe chronic periodontitis, a fact confirmed by other researchers [11,13].
More recently, Monteiro et al. [14] has shown that in individuals without coronary disease but with severe chronic periodontitis, the levels of triglycerides were high, while HDL cholesterol proved to be reduced, citing similarities with this study.
Using an animal model of chronic periodontitis-induced, some investigators have detected increased levels of LDL cholesterol in the peripheral level [15]. In the same direction, levels of LDL-cholesterol presented higher when compared to the severity of chronic periodontitis in our sample.
High concentrations of LDL-cholesterol and triglycerides and low levels of HDL cholesterol are well established as risk factors for coronary heart disease.
Patients who presented severe chronic periodontitis in our sample, and several clinical periodontal parameters in other studies showed high levels of triglycerides [16].
Specific bacteria that cause severe chronic periodontitis induce a response in the host, with the production and release of pro-inflammatory cytokines in gingival tissues, which flow into the blood flow, triggering a hepatic response of the liver, thereby raising levels of acute-phase proteins, mainly C-reactive protein [17].
Patients with great loss of gingival attachment and periodontal pockets present high rates of C-reactive protein in systemic circulation. When this protein is high in the systemic circulation it increases triglyceride levels [17].
Pussinen et al. [18] studying patients with severe chronic periodontitis, but without any systemic disease, observed statistically significant lower levels of HDL-cholesterol. Other researchers [16] also observed this inverse relationship with HDL-cholesterol, which corroborate our findings.
In patient with chronic infections with
Chlamydia pneumoniae, as diagnosed by their specific antibody titers, it has been also demonstrated an inverse relationship with HDL-cholesterol [19].
Poor glycemic control is widely known as a risk factor for severe chronic periodontitis [20], as well as chronic periodontitis in its various forms can deteriorate glycemic control [21], a fact observed in our sample.
The white cell count (Figure 5) represents a recent indicator of risk for coronary heart disease. This biochemical finding may be associated with unidentified infectious processes (chronic oral infections), which in our sample is higher in those with severe chronic periodontitis [22].
Studies in animal experiment demonstrated that intravenous administration of bacteria that cause chronic periodontitis increases the formation and calcification of atheromatous plaques [23].
Haraszthy et al. [3] examining 50 biopsies of aortic arteries with atheromas and using molecular biology techniques, found that 22 (44%) arteries were positive for several periodontal bacteria, including
Tannerella forsythia in 30%, Porphyromonas gingivalis in 26%,
Prevotella intermedia in 14% and
Agregatibacter actinomycetemcomitans in 18%, thus showing similarity with our sample.
In another study using molecular biology techniques [24], the researchers detected
Agregatibacter actinomycetemcomitans and
Prevotella intermedia in approximately 31% of multiple arterial specimens. In our sample, seventeen of coronary arteries with atherosclerosis studied, one-third (33%) had both periodontal bacteria.
Zaremba et al. [25], using 20 patients who underwent surgery for coronary artery patency and presenting diagnosis of severe chronic periodontitis, 13 periodontal bacteria were detected in obstructed coronary vessels. The authors noted that 10 of the 20 patients presented the same bacterial clones from periodontal pockets and in the coronary vessels.
Using the polymerase chain reaction, Aimetti et al. [26] found 33 external carotid atheromas to research of five species of bacteria that induce severe chronic periodontitis, in which the bacteria most prevalent (nearly 70%) was the
Tannerella forsythia, contrasting with our findings (12%).
Comparing the presence of periodontal bacteria in gingival pockets in patients with various levels of involvement of coronary arteries, Gotsman et al. [27] detected a higher prevalence of
Porphyromonas gingivalis in those with a greater number of vessels affected. In this direction, Stein et al. [28] detected using hybridization techniques, the prevalence of
Porphyromonas gingivalis in gingival pockets, in patients with acute myocardial infarction in comparison to their respective healthy controls.
However, in case-control study [29], the researchers found a higher prevalence of
Prevotella intermedia when comparing patients evaluated for coronary artery disease and their respective healthy controls.
Zhang et al. [30], using animal models and inoculating once a week for four, eight and twelve consecutive weeks the
Porphyromonas gingivalis in iliac arteries, they observed that the animals developed inflammation of the arterial intima and increases in other blood biochemical parameters related to inflammation throughout the study.
In our study, we detected the presence of pathogenic bacterial DNA that induces severe chronic periodontitis in about 60% of the coronary arteries studied.
Porphyromonas gingivalis was the periodontal pathogen most frequently detected (Figure 6). All specimens of internal mammary arteries were negative for the bacteria that cause severe chronic periodontitis.
Internal mammary artery is considered a vessel protected from the atherosclerotic process, thus being an ideal graft for the bridge and free from infection caused by periodontal microorganisms, in contrast to infections caused by
Chlamydia pneumoniae (35.29%).
The rate of detection of Chlamydia pneumoniae in our sample was 29.41% in the coronary artery, while in other studies the detection rate was around 40% [31], thus showing similar data with our study.
Based on these data, the hypothesis that the bacteria associated with severe chronic periodontitis can penetrate the ulcerated gingival epithelium, with access to blood flow and lodge in atheromatous plaques, as well as if these infections may alter the lipid profile in patients who suffered ischemic heart attack.
Therefore, further studies are needed to elucidate this relationship with large number of patients by means of longitudinal studies, and note mainly the frequency and incidence of microorganisms associated with gingival disease in periodontal pockets and in atheromatous plaques of patients with acute ischemic heart disease.
REFERENCES
1. Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol. 2000;2005;38:135-87.
2. Mattila KJ, Pussinen PJ, Paju S. Dental infections and cardiovascular diseases: a review. J Periodontol. 2005;76(11 Suppl):2085-8. [MedLine]
3. Haraszthy VI, Zambon JJ, Trevisan M, Zeid M, Genco RJ. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71(10):1554-60. [MedLine]
4. Berenson GS, Srinivasan SR, Bao W, Newman WP 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650-6. [MedLine]
5. Barter PJ, Rye KA. High density lipoproteins and coronary heart disease. Atherosclerosis. 1996;121(1):1-12. [MedLine]
6. Lindhe J, Ranney R, Lamster I, Charles A, Chung CP, Flemmig T, et al. Consensus report: chronic periodontitis. Ann Periodontol. 1999;4:38. [MedLine]
7. Scheffé H. A method for judging all contrasts in the analysis of variance. Biometrika. 1953;40:87-104.
8. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation on obesity. Geneva: World Health Organization;1997.
9. Katz J, Chaushu G, Sharabi Y. On the association between hypercholesterolemia, cardiovascular disease and severe periodontal disease. J Clin Periodontol. 2001;28(9):865-8. [MedLine]
10. Katz J, Flugelman MY, Goldberg A, Heft M. Association between periodontal pockets and elevated cholesterol and low density lipoprotein cholesterol levels. J Periodontol. 2002;73(5):494-500. [MedLine]
11. Cutler CW, Shinedling EA, Nunn M, Jotwani R, Kim BO, Nares S, et al. Association between periodontitis and hiperlipidemia: cause or effect? J Periodontol. 1999;70(12):1429-34. [MedLine]
12. Morita M, Horiuchi M, Kinoshita Y, Yamamoto T, Watanabe T. Relationship between blood triglyceride levels and periodontal status. Community Dent Health. 2004;21(1):32-6. [MedLine]
13. Lösche W, Karapetow F, Pohl A, Pohl C, Kocher T. Plasma lipid and blood glucose levels in patients with destructive periodontal disease. J Clin Periodontol. 2000; 27(8):537-41. [MedLine]
14. Monteiro AM, Jardini MA, Alves S, Giampaoli V, Aubin EC, Figueiredo Neto AM, et al. Cardiovascular disease parameters in periodontitis. J Periodontol. 2009;80(3):378-88. [MedLine]
15. Ebersole JL, Cappelli D, Mott G, Kesavalu L, Holt SC, Singer RE. Systemic manifestations of periodontitis in the non-human primate. J Periodontal Res. 1999;34(7):358-62. [MedLine]
16. Wu T, Trevisan M, Falkner KL, et al. Periodontal pathogens, serum lipids, and lipid peroxidation in a population-based sample. Circulation. 2001;103:1357a. [MedLine]
17. Yoshii S, Tsuboi S, Morita I, Takami Y, Adachi K, Inukai J, et al. Temporal association of elevated C-reactive protein and periodontal disease in men. J Periodontol. 2009;80(5):734-9. [MedLine]
18. Pussinen PJ, Vilkunna-Rautiainen T, Alfthan G, Palosuo T, Jauhiainen M, Sundvall J, et al. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler Thromb Vasc Biol. 2004;24(11):2174-80. [MedLine]
19. Murray LJ, O'Reilly DP, Ong GM, O'Neill C, Evans AE, Bamford KB. Chlamydia pneumoniae antibodies are associated with an atherogenic lipid profile. Heart. 1999;81(3):239-44. [MedLine]
20. Mealey BL, Oates TW; American Academy of Periodontology. Diabetes melitus and periodontal diseases. J Periodontol. 2006;77(8):1289-303. [MedLine]
21. Grossi SG, Genco RJ. Periodontal disease and diabetes mellitus: a two-way relationship. Ann Periodontol. 1998;3(1):51-61. [MedLine]
22. Cannon CP, McCabe CH, Wilcox RG, Bentley JH, Braunwald E. Association of white blood cell count with increased mortality in acute myocardial infarction and unstable angina pectoris. OPUS-TIMI 16 Investigators. Am J Cardiol. 2001; 87(5):636-9.
23. Brodala N, Merricks EP, Bellinger DA, Damrongsri D, Offenbacher S, Beck J, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2005;25(7):1446-51. [MedLine]
24. Taylor-Robinson D, Aduse-Opoku J, Sayed P, Slaney JM, Thomas BJ, Curtis MA. Oro-dental bacteria in various atherosclerotic arteries. Eur J Clin Microbiol Infect Dis. 2002;21(10):755-7. [MedLine]
25. Zaremba M, Górska R, Suwalski P, Kowalski J. Evaluation and the incidence of periodontitis-association bacteria in the atherosclerotic plaque of coronary blood vessels. J Periodontol. 2007;78(2):322-7. [MedLine]
26. Aimetti M, Romano F, Nessi F. Microbiologic analysis of periodontal pockets and carotid atheromatous plaques advanced chronic periodontitis patients. J Periodontol. 2007;78(9):1718-23. [MedLine]
27. Gotsman I, Lotan C, Soskolne WA, Rassovsky S, Pugatsch T, Lapidus L, et al. Periodontal destruction is associated with coronary artery disease and periodontal infection with acute coronary syndrome. J Periodontol. 2007;78(5):849-58. [MedLine]
28. Stein JM, Kuch B, Conrads G, Fickl S, Chrobot J, Schulz S, et al. Clinical periodontal and microbiologic parameters in patients with acute myocardial infarction. J Periodontol. 2009;80(10):1581-9. [MedLine]
29. Nonnenmacher C, Stelzel M, Susin C, Sattler AM, Schaefer JR, Maisch B, et al. Periodontal microbiota in patients with coronary artery disease measured by real-time polymerase chain reaction: a case-control study. J Periodontol. 2007;78(9):1724-30. [MedLine]
30. Zhang MZ, Li CL, Jiang YT, Jiang W, Sun Y, Shu R, et al. Porphyromonas gingivalis infection accelerates intimal thickening in iliac arteries in a balloon-injured rabbit model. J Periodontol 2008;79(7):1192-9. [MedLine]
31. Pucar A, Milasin J, Lekovic V,Vukadinovic M, Ristic M, Putnik S, et al. Correlation between atherosclerosis and periodontal putative pathogenic bacterial infections in coronary and internal mammary artery. J Periodontol. 2007;78(4):677-82. [MedLine]
Fernando J. de Oliveira is supported by Fundação de Assistência à Educação e Pesquisa (FAEP-UNICAMP).
Article receive on Tuesday, July 14, 2009






 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license