INTRODUCTION
Rupture of interventricular septum after acute myocardial infarction (AMI) is a mechanical complication associated with high mortality rates [1-3]. The use of thrombolytic agents appears to have reduced the incidence from 1% to 2% in the pre-thrombolytic period to 0.2% [1,2,4]. This complication occurs more frequently in the first week of AMI, typically three to five days after onset of ischemic symptoms [1,4,5]. The outcomes related to interventricular septum rupture in the pre-thrombolytic period were bad, with rates of in-hospital mortality of approximately 45% in patients treated surgically and 90% in clinically treated patients [1-3].
Despite the developments in the fast and effective treatment in the era of coronary reperfusion (with mechanical or chemical thrombolysis), data from SHOCK trials [6] and GUSTO-I [7] showed that the mortality of this complication has remained high, with rates of 87% and 73.8% respectively [6,7].
The Hospital Complex, University Hospital Osvaldo Cruz/Cardiac Emergency Hospital of Pernambuco (HUOC / PROCAPE), located on the campus of the University of Pernambuco (UPE) is a reference teaching hospital in Cardiology and Cardiovascular Surgery and covers assistance to the metropolitan area of Recife, municipalities in the interior of Pernambuco state and even neighboring states. The complex has a great demand for cardiac surgeries per month and it is still unknown the clinical profile and surgical outcomes of patients operated for ventricular septal rupture after acute myocardial infarction in the institution.
Considering these aspects, the authors present here a group of patients consecutively operated in the center abovementioned, with interventricular septum rupture after AMI, studying their preoperative clinical characteristics, associated complications and outcomes during hospitalization.
METHODS
We studied retrospectively patients operated in the Division of Cardiovascular Surgery at the Hospital Complex of the Oswaldo Cruz Hospital/Cardiac Emergency Hospital of Pernambuco - HUOC / PROCAPE, from January 1996 to June 2009, who developed ventricular septal rupture after AMI. The data were collected from the hospital records.
We reviewed the cases operated with the diagnosis of ventricular septal defect (VSD) after AMI: patients who had a heart murmur which appeared recently in the presence of myocardial infarction (recent or current) with transthoracic echocardiography and / or cardiac catheterization that showed the presence of interventricular shunt.
We evaluated the following independent variables: age, gender, hypertension, diabetes mellitus, tobacco use, dyslipidemia, obesity with body mass index greater than or equal to 30kg/m2, previous coronary artery disease, renal disease (on/off hemodialysis program), previous stroke, previous peripheral vascular disease, score in the EuroSCORE [8], type of infarction (with ST-segment elevation or not in the electrocardiogram - ECG), delta-T upon hospital admission (? 12 hours or> 12 hours) topography of infarction (locating the wall by ECG), type of treatment received at admission (conservative - without reperfusion therapy, chemical thrombolysis or primary percutaneous coronary intervention - PCI), coronary lesions (via cardiac catheterization), location of shunt (anterior, posterior or apical septum using transthoracic echocardiography), number of shunts (single or multiple), ventricular function (by ejection fraction in transthoracic echocardiography), time of onset of VSD (time elapsed between the onset of symptoms of myocardial ischemia until the identification of mechanical complications), complications and associated states (cardiogenic shock, stroke, acute renal failure requiring dialysis, respiratory failure, pulmonary edema, deep vein thrombosis, infections of any sort, arrhythmias), need for vasoactive drugs in the preoperative, use of intra-arctic balloon (IAB), time between diagnosis and surgery (in days), hemodynamic status upon arrival to surgery (stable or unstable), coronary artery bypass grafting (CABG) associated with VSD correction. The dependent variable was the outcome (discharge or death).
In order to analyze the data, we obtained absolute distributions, percentages and statistical measures: mean, standard deviation and coefficient of variation (descriptive statistics techniques). We used the following tests: Fisher's exact (provided that the conditions for using the chi-square were not checked) and Student's t test with equal or unequal variances. It is noteworthy that the verification of the hypothesis of equal variances was performed using the Levene's F test. The level of significance used in the decision of the statistical tests was 5.0%. Data were entered in the Excel spreadsheet and the statistical software used to obtain the statistical calculations was SPSS (
Statistical Package for the Social Sciences) version 15.
This study was approved by the Research Ethics Committee of the Hospital Complex, University Hospital Osvaldo Cruz / Cardiac Emergency Hospital of Pernambuco (CEP / HUOC / PROCAPE), protocol No. 095/2009.
RESULTS
We found 21 cases operated for ventricular septal rupture after AMI during the study period. The average age of patients was 62.81 years (SD ± 8.21 years), ranging from 49 to 82 years, and 61.9% (n = 13) were male and 38.1% (n = 8) female.
In the evaluation of clinical characteristics (Table 1), we observed the presence of: 52.4% (n = 11) hypertensive, 33.3% (n = 7) diabetic, 52.4% (n = 11) smokers, 19 % (n = 4) with dyslipidemia3., 14.3% (n = 3) obese, 14.3% (n = 3) with previous coronary disease, 9.5% (n = 2) patients with renal disease, 4 8% (n = 1) with previous stroke and no patient with peripheral vascular disease.

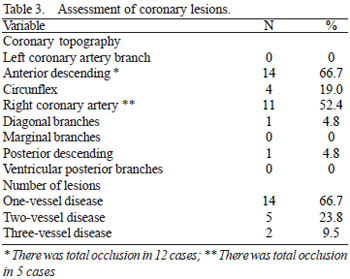
In the evaluation of acute infarction event (Table 2), we identified that 81% (n = 17) were victims of acute myocardial infarction with ST-segment elevation, being that 66.7% (14) had a delta-T of arrival > 12 hours. As for the walls affected by AMI, where 42.9% (n = 9) of the cases were of inferior wall (ST-segment elevation in leads D2, D3 and a VF on the ECG), 33.3% (n = 7) extensive anterior wall (ST-segment elevation in leads V1 to V6, with or without change in DI and a VL in ECG) and 23.8% (n = 5) in the anteroseptal wall (ST-segment elevation in leads V1 to V4 in ECG), totaling 57.1% (n = 12) involvement of the anterior wall. As for the treatment on admission, we observed that 66.7% (n = 14) received conservative treatment (no reperfusion therapy), 33.3% (n = 7) received chemical thrombolysis and no patient underwent primary percutaneous coronary intervention.
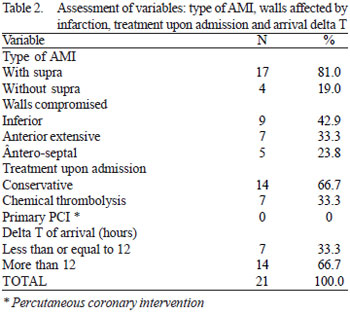
In the assessment of coronary lesions (Table 3) by means of cardiac catheterization, we observed the following frequencies: no lesion in left main coronary artery, 66.7% (n = 14) anterior descending artery (of which there were 12 such cases with total occlusion) 19% (n = 4), circumflex branch, 52.4% (n = 11) right coronary artery (of which there were five such cases with total occlusion), 4.8% (n = 1), diagonal branches, no lesions on marginal branches 4.8% (n = 1) posterior descending artery, and no lesion in the posterior ventricular branch. Regarding the number of coronary lesions, we identified 66.7% (n = 14) single-vessel disease, 23.8% (n = 5) two-vessel disease and 9.5% (n = 2) three-vessel disease.
As for the rupture of the interventricular septum, we noticed that the event occurred on average 4.8 days (SD ± 6.6 days) after AMI. Most patients (61.9%, n = 13) had a rupture within the first 2 days of AMI. Regarding the location of the shunt, 33.3% (n = 7) in the anterior septum, 33.3% (n = 7) in the posterior septum and 33.3% (n = 7) in the apical septum, being that in 95.2% (n = 20) the number of shunts was singular and in 4.8% (n = 1) multiple.
In the analysis of associated complications (Table 4), we observed the following frequencies: 57.1% (n = 12), cardiogenic shock, 0% (n = 0) stroke, 9.5% (n = 2) failure acute renal failure requiring dialysis, 9.5% (n = 2) respiratory failure, 4.8% (n = 1) acute pulmonary edema, 0% (n = 0), deep vein thrombosis, 9.5% (n = 2) infections of any sort and 9.5% (n = 2) arrhythmias. The cardiogenic shock complication was presented as a risk factor for adverse outcome (Table 5), the death rate was 100% in patients with cardiogenic shock versus 22.2% in non-carriers of cardiogenic shock (
P <0.001).
In the evaluation of left ventricular function, there was a mean ejection fraction of 50.6% (SD ± 12.3%). Patients that survived the surgery had a higher mean ejection fraction compared to patients who progressed to death as the outcome (Table 6), being this difference statistically significant (survival: 66.29% ± 4.61% versus deaths: 42.71% ± 4.79%,
P <0.001).
The average time between diagnosis and surgery was 7.8 days (SD ± 6.4 days). Patients that survived the surgery had a higher mean time between diagnosis and major surgery compared to patients who progressed to death as the outcome (Table 6), being this difference statistically significant (survival: 11.86 ± 8.07 days versus deaths: 5.79 ± 4.42 days,
P = 0.036).
All study patients were classified as high surgical risk considering the EuroSCORE (score e" 6), and the average score of 9.5 points (SD ± 2.8). Patients that survived the surgery had a lower mean score in the EuroSCORE compared to patients who progressed to death as the outcome (Table 6), this difference was statistically significant (survival 6.57 ± 0.53 points versus deaths 10.93 ± 2.23 points,
P <0.001).
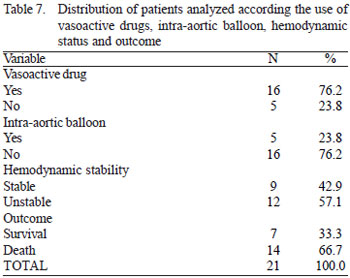
As for the clinical condition before entering the operating room, 76.2% (n = 16) required the use of vasoactive drugs, 23.8% (n = 5) used an intra-arctic balloon and 57.1% (n = 12) were considered hemodynamically unstable (Table 7). The need for vasoactive drugs preoperatively presented as a risk factor for death as the outcome (see Table 8; rate of 81.3% in the group with vasoactive drugs versus 20% in the group without vasoactive drugs, P = 0.025). It was observed that the variable hemodynamic instability was also a risk factor for adverse outcome (see Table 8, the death rate was 100% in the hemodynamically unstable group versus 22.2% in the hemodynamically stable group,
P <0.001).
No patient underwent CABG associated with VSD correction.
There was high rate of in-hospital mortality (66.7%, n = 14), with only 33.3% (n = 7) of survival (Table 7).
DISCUSSION
The mean age reported by patients in this study (62.81 years) does not seem to be consistent with the studies SHOCK [6] and GUSTO-I [7], the latter being the older patients (average 72 and 71.8 years respectively). The age of our patients is more akin to the studies of the pre-thrombolytic era [9-11]. Most of our patients were men (61.9%, n = 13), a fact that is consistent with other studies, however, in contrast to the SHOCK study [6] and GUSTO-I [7], in which predominated female. The mean time between the onset of AMI to rupture of the interventricular septum was 4.8 days in this study and more than half of patients developed this complication within the first 2 days. This observation may indicate that the rupture may occur earlier than is indicated in studies of the pre-thrombolytic era [1,5,12-14]. Although thrombolytic therapy reduces infarct extension, reperfusion may promote myocardial hemorrhage and myocardial dissection, accelerating the risk of rupture [15]. Early diagnosis of this complication reflects rapid access to echocardiography in the Hospital Complex HUOC/PROCAPE, since it is a tertiary hospital specialized in Cardiology and Cardiovascular Surgery.
As shown in other studies, this showed a predominance of single-vessel coronary artery disease (66.7%, n = 14) with total occlusion of the artery responsible for infarction in more than half of patients. It is reported that limited coronary artery diseases represented by not extensive and specific injuries lead to poorly developed collateral circulation, leaving the wall hit by the acute event without any protection, making it more susceptible to rupture [7.16 to 18].
It has been reported that anterior wall infarcts are more likely to complicate with rupture of the interventricular septum compared with inferior wall infarction. [5,14,19]. This study, confirms a slight predominance of anterior wall myocardial infarction (57.1%, n = 12) compared to inferior wall infarction in patients complicated with ventricular septal rupture after myocardial infarction.
Despite being admitted to tertiary care hospital, none of the patients received reperfusion therapy with primary PCI. This occurred because most patients (66.7%, n = 14) have arrived after 12 hours of symptoms and/or had no indication of reperfusion therapy (because they had AMI without ST-segment elevation). Among those who arrived less than 12 hours of onset of symptoms, all had AMI with ST-segment elevation, receiving reperfusion therapy with thrombolytics. It is worth a brief comment on the relationship between thrombolytic and development of ventricular septal rupture after AMI.
Westaby et al. [21] studied 29 patients who developed ventricular septal rupture after AMI (26 of which were operated), showed that the mean interval between the onset of symptoms of acute myocardial ischemia and the development of septal rupture was 24 hours for those treated with thrombolytics (all with streptokinase) and 6 days for those who received conservative treatment. In the same study, the macroscopic observation of the infarcted myocardium showed muscle bundles dissected by non-clottable blood due to the thrombolytic treatment, along with the histological features of reperfusion injury. The authors concluded that early thrombolysis led to the collapse of the interventricular septum after acute myocardial infarction.
More than half of patients developed cardiogenic shock and a little more than three-quarters received inotropic support with vasoactive drugs, which indicates that our sample consisted of more severe patients compared to other studies [7,15,19], in which only one third of patients progressed to cardiogenic shock and up to two thirds required vasoactive drugs. The simple need for inotropic support with vasoactive drugs was associated with increased risk for adverse outcome (Table 8, the death rate was 81.3% in the group with vasoactive drugs versus 20% in the group without vasoactive drugs,
P = 0.025). It should not be concluded that vasoactive drugs are the cause of increased mortality, there is certainly a confounding factor related to the hemodynamic stability of these patients. Who needs inotropic support with vasoactive drugs? Answer: hemodynamically unstable patients. This one (hemodynamic instability) is the true cause of increased mortality. One must observe (Table 8) that the death rate was 100% in the hemodynamically unstable group versus 22.2% in hemodynamically stable group, being this difference statistically significant (
P <0.001).
Curiously, despite the severity of the patients in this series in comparison to these same studies [7,15,19], being used in the latter intra-arctic balloon in about half of patients, the IAB was used in less than a quarter of patients in our study. This fact was, probably, due to the recent acquisition of IAB in the service not being available for use before 2008. Although there are no definitive studies showing that the IAB improves survival in this specific group of patients, it is widely accepted that its use is favorable for the treatment of ventricular septal rupture after AMI [15]. The IAB reduces the left ventricular afterload, it decreases the flow of the left-right shunt and improves coronary perfusion, in a way that stabilizes and improves the clinical and hemodynamic conditions [15] before surgery.
Similar to other authors [15], we observed in this study that patients who survived surgery were operated, on average, later compared to those with adverse outcome, this difference being statistically significant (time between diagnosis and surgery survivors: 11, 86 ± 8.07 days versus deaths: 5.79 ± 4.42 days,
P = 0.036). We shall consider a bit this observation. The fragile necrotic myocardium is a major concern when it is decided by early surgery. From a technical standpoint, the best time to perform surgery is after the fibrotic healing of the necrotic muscle, when it becomes less brittle, better supporting the tension of the sutures. [15]. However, in a histological study [20], it was demonstrated that the proliferation of the connective tissue was not present until the third week after infarction. Moreover, a large proportion of patients can not postpone the surgery because they develop severe heart failure, cardiogenic shock and multiple organ dysfunctions [15].
It is worth noticing that in our series, progression to cardiogenic shock was presented as a risk factor for adverse outcome, resulting in a mortality rate of 100% versus 22.2% in non-carriers of cardiogenic shock (
P <0.001), a result similar to findings in other studies [19]. Hemodynamic deterioration before surgery and evolution to cardiogenic shock are known as strong predictors of early mortality [7.19]. In theory, a logical step to get better outcomes would be to operate patients immediately after the diagnosis, before the hemodynamic deterioration onset [15].
David et al. [22] demonstrated that the reduced left ventricular function was a predictor of early mortality. Confirming this previous observation, we observed in our series that patients with adverse outcomes had a lower mean left ventricular function (measured by ejection fraction) compared to survivors (Table 6), this difference was statistically significant (survivors: 66.29 % ± 4.61% versus deaths: 42.71% ± 4.79%,
P <0.001).
All the study patients were classified as high surgical risk considering the EuroSCORE (score ? 6). This fact was expected, considering the very fact that the patient had a VSD after AMI has already registered four points in the score. It was observed that patients with adverse outcome had a higher mean score (statistically significant) compared to patients who had a successful outcome (survival: 6.57 ± 0.53 versus deaths: 10.93 ± 2.23,
P <0.001, Table 6). Other Brazilian studies [23,24] validated this system in CABG and heart valve surgery as a satisfactory predictor of operative mortality being: the higher the surgical risk, the higher scores.
No patient in our series underwent CABG associated with the VSD correction. This was probably due to the observance of high surgical risk to which the patients were subject, considering the addition of CABG to the correction of the VSD (with increased surgical time, prolonged cardiopulmonary bypass with poor hemodynamic conditions of patients in general when entering the operating room) as an additional factor for increased operative mortality. The need for concomitant CABG is a controversial issue. Some series suggest that concomitant revascularization may improve the late survival [25,26] while others failed to show any definite benefit from concomitant coronary artery bypass surgery [27,28].
We observed a high rate of in-hospital mortality (66.7%, n = 14). This rate was even higher than that observed in GUSTO-I (53%); nevertheless, we must remember that our patients were more severe than the patients in the latter, since our study showed a higher percentage of hemodynamically unstable patients, with greater need for vasoactive drugs and greater progression to cardiogenic shock before surgery.
LIMITATIONS
The number of patients in this sample is small and, although several have shown statistically significant results, the interpretations shall take this into account. This study captured for analysis only patients who eventually underwent surgery, with a loss of patients who never underwent surgery for having evolved to death even before the surgery was performed. Although no positive results were observed for the IAB, the authors dare not infer that it does not bring benefits to this group of patients, considering that it was only used in less than one quarter of individuals in our study, the fact is, probably, due to the recent acquisition of this technology in the service, not being available for use before 2008.
Another aspect that must be noted regards the definition of two variables that were significantly associated in the study: hemodynamic stability and cardiogenic shock. Since this study is based on report review, the authors do not actually know what criteria the professionals who attended the patients used to define their hemodynamic stability or the presence of cardiogenic shock, so that at the time of data collection, there was no way to know which criteria were these and whether they were homogeneous regarding the definition, a defect inherent to studies using secondary data. The authors relied on the presence of the terms "hemodynamically stable", "hemodynamically unstable" and "cardiogenic shock" registered in the reports.
CONCLUSION
We draw the following clinical-surgical profile of the ventricular septal rupture after AMI in our series:
1. Most patients had one-vessel coronary disease;
2. There is a slight predominance of involvement of the anterior wall acute event;
3. The following factors were associated with higher rates of mortality, need for vasoactive drugs in the pre-operative period, hemodynamic instability and cardiogenic shock;
4. Patients who develop adverse outcomes have lower left ventricular function (with lower ejection fractions) and higher score in the EuroSCORE;
5. Patients operated for correction of this condition had a high mortality rate.
REFERENCES
1. Topaz O, Taylor AL. Interventricular septal rupture complicating acute myocardial infarction: from pathophysiologic features to the role of invasive and noninvasive diagnostic modalities in current management. Am J Med. 1992;93(6):683-8. [MedLine]
2. Heitmiller R, Jacobs ML, Daggett WM. Surgical management of postinfarction ventricular septal rupture. Ann Thorac Surg. 1986;41(6):683-91. [MedLine]
3. Davies RH, Dawkins KD, Skillington PD, Lewington V, Monro JL, Lamb RK, et al. Late functional results after surgical closure of acquired ventricular septal defect. J Thorac Cardiovasc Surg. 1993;106(4):592-8. [MedLine]
4. Moore CA, Nygaard TW, Kaiser DL, Cooper AA, Gibson RS. Postinfarction ventricular septal rupture: the importance of location of infarction and right ventricular function in determining survival. Circulation. 1986;74(1):45-55. [MedLine]
5. Edwards BS, Edwards WD, Edwards JE. Ventricular septal rupture complicating acute myocardial infarction: identification of simple and complex types in 53 autopsied hearts. Am J Cardiol. 1984;54(10):1201-5. [MedLine]
6. Menon V, Webb JG, Hillis LD, Sleeper LA, Abboud R, Dzavik V, et al. Outcome and profile of ventricular septal rupture with cardiogenic shock after myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries in cardiogenic shocK? J Am Coll Cardiol. 2000;36(3 Suppl A):1110-6. [MedLine]
7. Crenshaw BS, Granger CB, Birnbaum Y, Pieper KS, Morris DC, Kleiman NS, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation. 2000;101(1):27-32. [MedLine]
8. Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16(1):9-13. [MedLine]
9. Cummings RG, Califf R, Jones RN, Reimer KA, Kong YH, Lowe JE. Correlates of survival in patients with postinfarction ventricular septal defect. Ann Thorac Surg. 1989;47(6):824-30. [MedLine]
10. Held AC, Cole PL, Lipton B, Gore JM, Antman EM, Hockman JS, et al. Rupture of the interventricular septum complicating acute myocardial infarction: a multicenter analysis of clinical findings and outcome. Am Heart J. 1988;116(5 Pt 1):1330-6. [MedLine]
11. Feneley MP, Chang VP, O'Rourke MF. Myocardial rupture after acute myocardial infarction. Ten year review. Br Heart J. 1983;49(6):550-6. [MedLine]
12. Radford MJ, Johnson RA, Daggett WM Jr, Fallon JT, Buckley MJ, Gold HK, et al. Ventricular septal rupture: a review of clinical and physiologic features and an analysis of survival. Circulation. 1981;64(3):545-53. [MedLine]
13. Deville C, Fontan F, Chevalier JM, Madonna F, Ebner A, Besse P. Surgery of post-infarction ventricular septal defect: risk factors for hospital death and long-term results. Eur J Cardiothorac Surg. 1991;5(4):167-74.
14. Lemery R, Smith HC, Giuliani ER, Gersh BJ. Prognosis in rupture of the ventricular septum after acute myocardial infarction and role of early surgical intervention. Am J Cardiol. 1992;70(2):147-51. [MedLine]
15. Poulsen SH, Praestholm M, Munk K, Wierup P, Egeblad H, Nielsen-Kudsk JE. Ventricular septal rupture complicating acute myocardial infarction: clinical characteristics and contemporary outcome. Ann Thorac Surg. 2008;85(5):1591-6. [MedLine]
16. Figueras J, Cortadellas J, Soler-Soler J. Comparison of ventricular septal and left ventricular free wall rupture in acute myocardial infarction. Am J Cardiol. 1998;81(4): 495-7. [MedLine]
17. Prêtre R, Rickli H, Ye Q, Benedikt P, Turina MI. Frequency of collateral blood flow in the infarct-related coronary artery in rupture of the ventricular septum after acute myocardial infarction. Am J Cardiol. 2000;85(4):497-9.
18. Barreto FE, Castelli JB. Homem de 88 anos de idade com edema agudo dos pulmões, choque cardiogênico e novo sopro holossistólico de aparecimento recente. Arq Bras Cardiol. 2005;85(4):290-4. [MedLine]
19. Deja MA, Szostek J, Widenka K, Szafron B, Spyt TJ, Hickey MS, et al. Post infarction ventricular septal defect - can we do better? Eur J Cardiothorac Surg. 2000;18(2):194-201. [MedLine]
20. Fishbein MC, Maclean D, Maroko PR. The histopathologic evolution of myocardial infarction. Chest. 1978;73(6):843-9. [MedLine]
21. Westaby S, Parry A, Ormerod O, Gooneratne P, Pillai R. Thrombolysis and postinfarction ventricular septal rupture. J Thorac Cardiovasc Surg. 1992;104(6):1506-9. [MedLine]
22. David TE, Dale L, Sun Z. Postinfarction ventricular septal rupture: repair by endocardial patch with infarct exclusion. J Thorac Cardiovasc Surg. 1995;110(5):1315-22. [MedLine]
23. Moraes F, Duarte C, Cardoso E, Tenório E, Pereira V, Lampreia D, et al. Avaliação do EuroSCORE como preditor de mortalidade em cirurgia de revascularização miocárdica no Instituto do Coração de Pernambuco. Rev Bras Cir Cardiovasc. 2006;21(1):29-34. View article
24. Andrade ING, Moraes Neto FR, Oliveira JPSP, Silva ITC, Andrade TG, Moraes CR. Avaliação do EuroSCORE como preditor de mortalidade em cirurgia cardíaca valvar no Instituto do Coração de Pernambuco. Rev Bras Cir Cardiovasc. 2010;25(1):11-8. [MedLine] View article
25. Muehrcke DD, Daggett WM Jr, Buckley MJ, Akins CW, Hilgenberg AD, Austen WG. Postinfarct ventricular septal defect repair: effect of coronary artery bypass grafting. Ann Thorac Surg. 1992;54(5):876-82.
26. Cox FF, Plokker HW, Morshuis WJ, Kelder JC, Vermeulen FE. Importance of coronary revascularization for late survival after postinfarction ventricular septal rupture. A reason to perform coronary angiography prior to surgery. Eur Heart J. 1996;17(12):1841-5. [MedLine]
27. Dalrymple-Hay MJ, Monro JL, Livesey SA, Lamb RK. Postinfarction ventricular septal rupture: the Wessex experience. Semin Thorac Cardiovasc Surg. 1998;10(2):111-6. [MedLine]
28. Loisance DY, Lordez JM, Deleuze PH, Dubois-Rande JL, Lellouche D, Cachera JP. Acute postinfarction septal rupture: long-term results. Ann Thorac Surg. 1991;52(3):474-8. [MedLine]
Article receive on Sunday, March 28, 2010



 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license