INTRODUCTION
Rheumatic disease is at epidemic levels in poor regions, giving a high prevalence (2/1000 inhabitants) and affecting children and teenagers and even young adults. The disease normally involves the heart muscle and almost always leaving valvar sequels [1].
Rheumatic mitral insufficiency is, without doubt, the most common injury. In order to correct it using prostheses in children and adolescents there are limitations. These limitations are due to either socioeconomic reasons, as using metallic prostheses requires the maintenance of long-term anticoagulation, or due to the high morbidity rate, when biological prostheses are used owing to their early degeneration. The technique of concentric mitral annuloplasty without the use of a prosthesis was developed in our institution but it is a procedure which is infrequently reported in the literature.
METHOD
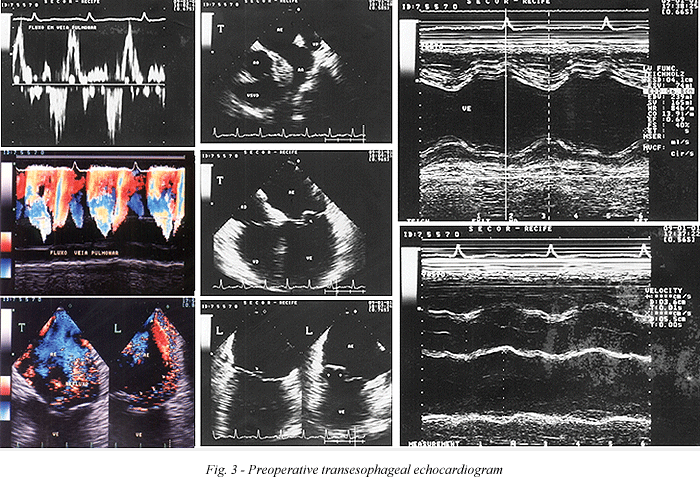
Between February 1986 and February 2001, 790 procedures involving the mitral valve were performed in the Cardiovascular and Thoracic Surgery Department of the Real Hospital Português de Beneficência in Pernambuco, Brazil. Forty-one of the operations were in children or under twenty-year-old adolescents (mean 9.7 years). Twenty (48.7%) of the patients were female and 21 (51.3%) were male. Rheumatic fever was responsible for the injury in 92.6% and myxomatous degeneration in 7.4% of the patients. In the preoperative period, 22 (53.6%) patients were in functional class III and 19 (46.4%) in functional class IV, following repetitive rheumatic fever events. Some patients were operated on in the acute phase of the disease, aggravated by malnutrition (Table 1).
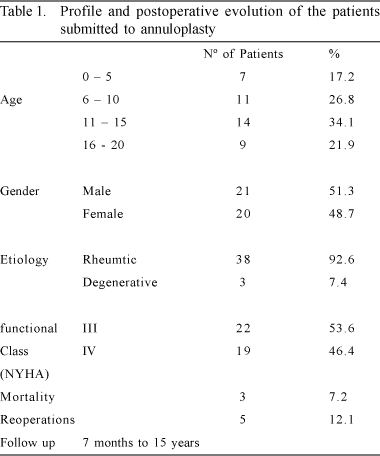
The surgical technique employed was concentric mitral annuloplasty without the use of a prosthesis, which is performed by anchoring two 2-0 Ethibond threads on a Teflon felt, taking as a reference point for the beginning of the circular running stitches, the middle of the annular implantation of the anterior leaflet. From there, with one thread a running stitch is made in an anti-clockwise direction, deep into the annulus to the mid-point of the annular implantation of the mural or posterior leaflet. Utilizing the other 2-0 Ethibond thread, we followed the same route, completing half of the mitral valve with two layers of running stitches. Another 2-0 Ethibond thread is passed through the Teflon felt which serves as anchoring for the first sutures. Each of the thread loops is sutured deeply in the annulus, this time in a clockwise direction so that both threads reach the medial portion of the posterior leaflet. Another Teflon felt patch is used to anchor the four threads. A previous comparison of the anterior leaflet enables the correct choice of diameter for the atrioventricular obturator (Nº 23, 25, or 27), which is inserted behind the mitral valve. The two double running stitches are tightened like a tourniquet, adjusting the new mitral valve to the diameter of the obturator. Tying off the threads completes the concentric annuloplasty procedure (Figures 1 & 2).

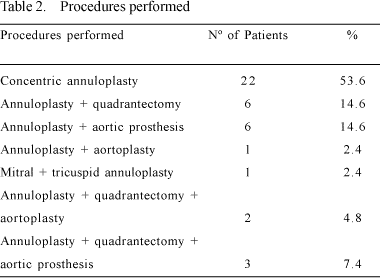
Quadrangular resection and posteromedial plicature of the mitral valve is a classical procedure already described [2,3], which was also used. Its association to concentric annuloplasty is an extra resource of the procedure. The application of quadrangular resection as an associated procedure to concentric annuloplasty was made in 11 (26.2%) patients - Table 2. Concentric annuloplasty is indicated in all cases where there is dilation of the mitral valve with whole or thickened cuspids (Figure 3).
All the patients were operated on under cardiopulmonary bypass using cannulation of the two vena cavas and the ascending aorta, moderate hypothermia, myocardial protection with normothermia sanguineous cardioplegia (repeated when necessary) complemented with 0.9% saline solution ice in the pericardial cavity. The mean time of perfusion was 30.5 minutes and 21 minutes when only the concentric annuloplasty was performed. The average time of aortic clamping was 22 minutes and 16 minutes for concentric annuloplasty in isolation. In the postoperative period, care was taken in the routine established for heart surgery using cardiopulmonary bypass.
RESULTS
One (2.4%) death occurred in the immediate postoperative period owing to myocardial failure and two deaths following reoperations to correct failure of the previously performed aortoplasty (12 and 18 months after the initial surgery). Four reoperations were performed, two to correct aortic dysfunction and two other patients who evolved with mitral stenosis (4 and 11 years) after annuloplasty owing to progression to rheumatic diseases. One was first operated at 23 months and again at 11 years.
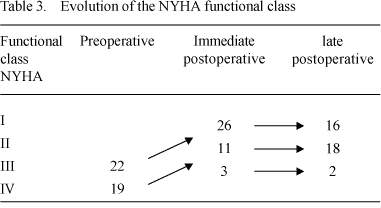
Nineteen (46.5%) patients who had functional class IV and 22 (53.6%) patients who had functional class III in the preoperative period improved to functional classes I and II. Only one patient who had functional class III in the preoperative period continued as class III - Table 3.
The actuarial survival curve is 92.7%, with 86.8% of the patients reoperation-free in a period of from 7 months to 15 years (Figure 4).
COMMENTS
Poverty facilitates the high incidence of rheumatic fever in our communities, increasing the appearance of valvar sequels, involving one or more heart valves in children and adolescents.
Mitral valve insufficiency in isolation or associated with aortic valve insufficiency constitutes the most common injury and is responsible for an early clinical picture of severe congestive heart failure. In the preoperative period this group of patients is classified as functional classes III and IV of the NYHA classification. This group accounts for a large number of valve surgeries.
The statement that mitral valve dilation is not responsible for mitral failure [4] was not verified in our experience. In our cohort, mitral insufficiency is the most common sequel of rheumatic fever, representing 92.6% of this series, which began in the phase of rheumatic carditis, causing an increase in the cardiothoracic index, responsible for mitral annulus dilation and, consequently, mitral insufficiency. This does not necessarily imply the existence of structural alterations of the chords and leaflets.
The repetitive episodes of rheumatic fever and the clinical difficulty to control congestive heart failure in this group lead these patients to a surgical solution, even the younger patients. Our youngest patient was 1 year and 11 months old and the oldest was 20 years old (mean of 9.7 years).
In the correction of rheumatic mitral insufficiency of children and adolescents, if the surgeon chooses to utilize a valvar prosthesis he will face dilemmas of socioeconomic origins. If the surgeon uses a metallic prosthesis, the difficulties involved with maintaining anticoagulation must be considered and on using a biological prosthesis, the elevated mortality associated to early degeneration must be remembered.
Hence, there is a necessity to give value to surgical techniques which preserve the native valve. Undeniably, CARPENTIER et al. [5] were responsible for the universal spread of the technique of valvar preservation using reconstruction.
Curiously, all the different techniques of plasty proposed for the correction of mitral insufficiency have as their aim, to reduce the diameter of the mitral annulus [6-13]. Antunes [10] justified the use of a rigid annulus to re-establish the diameter, supplying support and the form of the mitral valve and stabilizing the plasty performed.
The technique of concentric annuloplasty without the use of a prosthesis described here, associated or not with quadrantectomy with plicature of the posteromedial annulus, aims at stabilizing the functional mechanism of the mitral valve, adjusting the coaptation of its leaflets, without the necessity of shortening, lengthening or transferring chords. This technique was used in 41 children and adolescents, who presented with an actuarial survival curve of 92.7% with 86.8% of patients reoperation-free in the period from 7 months to 15 years.
CONCLUSION
We can conclude that correcting mitral insufficiency predominantly caused by rheumatic disease employing concentric annuloplasty as we have recommend, associated or not to quadrangular resection and posteromedial plicature of the mitral valve, is easy to perform and, more importantly of a low cost.
BIBLIOGRAPHIC REFERENCES
1. Gus I, Zaslavsky C, Seguer JM, Machado RS. Epidemiologia da febre reumática aguda: estudo local. Arq Bras Cardiol 1995; 65:321-5.
2. Wooler GA, Nixon PGF, Grimshan VA, Watson DA. Experience with repair of the mitral volume in incompetence. Thorax 1962; 17:49-57.
3. Reed GE, Tice DA, Clauss RH. Asymmetric exaggerated mitral annuloplasty: repair of mitral insufficiency with hemodynamic predictability. J Thorac Cardiovasc Surg 1965; 49:752-61.
4. Bulkley BH, Roberts WC. Dilatation of mitral annulus: a rare case of mitral regurgitation. Am J Med 1975; 59:457-63.
5. Carpentier A, Deloche A, Dauptain J, Soyer R, Blondeau P, Piwnica A et al. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J Thorac Cardiovasc Surg 1971; 61:1-13.
[ Medline ]
6. Arruda MB, Moraes CR, Lagreca JR et al. Anuloplastia mitral em crianças e adolescentes. Rev Col Bras Cir 1974; 9:219-22.
7. Merendino KA, Thomas GI, Jesseph JE, Herron PW, Winterscheid LC, Vetto RR. The open correction of rheumatic mitral regurgitation and/or stenosis with special reference to regurgitation treated by posteromedial annuloplasty utilizing a pump-oxygenator. Ann Surg 1959; 150:5-22.
8. Gregori Jr. F, Silva SS, Baba X, Queiroz LT, Takeda R, Façanha LA et al. Um novo modelo de anel protético para pacientes com insuficiência mitral: relato de 2 casos. Arq Bras Cardiol 1988; 50:417-20.
[ Medline ] [ Lilacs ]
9. Burr LH, Krayenbuhl C, Sutton MS. The mitral plication suture: a new technique of mitral valve repair. J Thorac Cardiovasc Surg 1977; 73:589-95.
[ Medline ]
10. Antunes JM. Mitral valve repair. Germany: R.S. Schultz; 1989. p.91.
11. Pomerantzeff PMA, Amato M, Stolf NAG, Marcial MB, Grinberg M, Pileggi F et al. Experiência com plástica de valva mitral. Arq Bras Cardiol 1985; 45 (suppl 1): 156.
12. Braile DM, Ardito RV, Pinto GH, Santos JLV, Zaiantchick M, Souza DRS et al. Plástica mitral. Rev Bras Cir Cardiovasc 1990; 5:86-98.
[ Lilacs ]
13. Carpentier A, Chauvaud S, Fabiani JN, Deloche A, Relland J, Lessana A et al. Reconstructive surgery of mitral valve incompetence: ten years appraisal. J Thorac Cardiovasc Surg 1980; 79:338-48.
[ Medline ]





 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license