Objective: To describe the adverse effects that occur during and after percutaneous transluminal coronary angioplasty (PTCA) possibly related to the reuse of medical equipment. An additional objective is to quantify and identify the reasons of discard in respect to the brand-new and reuse of medical equipment.
Method: Sixty patients were studied (48.3% with unstable angina, 45% with acute myocardial infarction and 6.7% with other diagnoses). During the procedure and stay in the Intensive Coronary Unit, the occurrence of fever, hypotension or hypertension, chills, sudoresis, bleeding, nausea and vomits were observed. Seven products were evaluated: catheter introducer, catheter guides (0.35 and 0.014), catheter balloons for angioplasty, indeflators and manifolds. In total, 76 brand-new and 410 reused apparatuses were studied to verify the occurrence of discard, whether this happened before or during the procedure and for what reasons. P-values 0.05 were considered signicant.
Results: Twenty-six patients presented adverse effects. Hypotension was the most common seen in 11(18.3%) cases. There was no significant association between this adverse effect and reuse or not of the equipment. Three brand-new products and 55 of the reused products were discarded as they were incomplete.
Conclusion: The adverse effects presented by patients submitted to coronary vessel angioplasty were not associated to the reuse of the medical equipment. The integrity and functionality were the main reasons of discard.
bjetivo: Descrever os eventos adversos ocorridos durante e após angioplastia coronária (ATC), possivelmente relacionados ao reuso de produtos médico-hospitalares, além de quantificar e identificar os motivos de descarte em relação ao primeiro uso e ao reuso.
Método: Foram estudados 60 pacientes, sendo que 29 (48,3%) apresentavam angina instável, 27 (45%) IAM e quatro (6,7%) outros diagnósticos. Durante o procedimento e na permanência na Unidade Intensiva Coronariana, atentou-se à possibilidade de ocorrência dos eventos adversos febre, hipertensão, hipotensão, calafrios, sudorese, sangramento, náuseas e vômitos. Foram avaliados sete produtos médico-hospitalares: introdutor, cateter-guia, fio-guia 0.35, fio-guia 0.014, cateter- balão para angioplastia, seringa com manômetro para insuflar balão (indeflator) e torneira de três vias (manifold). No total de produtos (76 de primeiro uso e 410 reprocessados), verificou-se se houve descarte e se isto ocorreu antes ou durante o procedimento e quais os motivos para tanto. Utilizou-se o teste Qui Quadrado, admitindo-se erro alfa de 5%.
Resultados: Vinte e seis pacientes apresentaram eventos adversos. A hipotensão foi o evento mais prevalente e ocorreu em 11(18,3%) casos. Não houve, porém, significância estatística entre o evento adverso hipotensão e reuso ou não dos produtos médico-hospitalares. Por não estarem íntegros, foram descartados três produtos de primeiro uso e 55 produtos dos reutilizados.
Conclusão: Os eventos adversos apresentados pelos pacientes submetidos à angioplastia não estão associados ao reuso dos produtos médico-hospitalares. A integridade e funcionalidade foram os motivos principais de descarte.
INTRODUCTION
Hemodynamic catheters, used for examinations, diagnoses and heart interventions including angioplasty, are among the most commonly recycled disposable medical products around the world. Concern on the reuse of these devices has been creating many challenges for the professionals who use them.
The technical difficulties to recycle products and adverse events are important questions in the decision to reutilize medical products for angioplasty [1].
In the current state of the health services, where diagnostic and therapeutic procedures using invasive catheters predominate, the processes of cleaning, disinfection and sterilization of medical products are of extreme relevance in the control of hospital infection [2].
The fragility, integrity and functionality of these products have been the subject of several publications. Many disposable surgical equipment manufacturers have investigated the deterioration of articles during recycling, in particular, for articles made out of polymers denominated PVC [3].
If the devices are used repeatedly, it is expected that gradually they will lose their original functionality. The physical, optical, mechanical and electronic properties of devices normally deteriorate with continuous use. The processes of cleaning with detergents and other chemical substances were not considered during their manufacture and can alter the composition of polymers, as contact with organic solvents promotes the extraction of additives incorporated in the formulation of polyvinylchloride compounds which, consequently, become more flexible and more fragile [2].
The progressive degradation of the composition of polymers of plastics, compromise the performance and the functionality of devices. The bioengineering sector of hospitals can provide conditions to test the functionality of these articles using methods to inspect their safety and performance [4].
It can be supposed that the reutilization of products is a causal factor of adverse clinical events after procedures. Thus the objectives of this study were: to describe the adverse clinical events during and after angioplasty, which are possibly connected to the reuse of medical products and to quantify and identify the reasons for rejecting products in respect to its original and subsequent uses.
METHOD
Sixty patients submitted to elective coronary transluminal angioplasty (CTA) [5,6] participated in this study with patients undergoing emergency procedures being excluded.
The CTA procedure was performed under local anesthesia using the Judkins' technique (femoral percutaneous) [7]. Of this group, 33 (55%) were men. The ages varied from 42 to 88 years old (mean 63 ± 10 years).
The mean weight was 70 ± 12.8 kg and the mean height was 1.6 ± 0.1 m giving a average body mass index (BMI) [8,9] for the 60 patients of 25.6 ± 3.5 kg/m2.
According to anamnesis, 22 patients (36.7%) had arterial hypertension, 10 (16.6%) had diabetes, 15 (25%) were smokers and eight (13.3%) were alcoholics.
Of the 60 patients, 29 (48.3%) presented with unstable angina, 27 (45%) with myocardial infarction and four (6.7%) with other diagnoses.
The mean hospitalization of the patients was seven days and the median was two days with the patients remaining for the first 24 hours after the procedure in the Coronary Intensive Care Unit (UCOR).
The mean time of the angioplasty procedure was 35 minutes and radioscopy (radiation) time, six minutes.
During the procedure and the stay in UCOR, adverse events [10] presented by the patients that may have been connected in any way with the use of medical products were recorded. The patients were monitored in respect to fever, hypertension, hypotension, chills, sudoresis, bleeding, nauseas and vomiting.
Fever [11] was considered at temperatures of 38ºC or higher, hypertension [12,13] when the systolic pressure was greater than 140 mmHg or the diastolic pressure greater than 90 mmHg and hypotension when the systolic pressure was less than 100 mmHg.
At the entry site of the catheter, excessive blood loss that could lead to the formation of hematomas was occasionally seen until the catheter was removed [7].
Categorical variables were analyzed using the chi squared test. Discreet quantitative variables were analyzed using the non-parametric Mann-Whitney test. An alpha error of 5% was considered acceptable and so significant differences were considered with p-values < 0.05.
Seven types of medical products involved in the procedure were analyzed giving a total of 468 items, with 76 (15.64%) first-time use and 410 (84.5%) recycled [14-16]. A check was made to see if the products were discarded before or during the procedure and for what reason.
The introducer sheaf, guide catheter, 0.35 guide wire, 0.014 guide wire, balloon catheter for angioplasty were considered critical items and the indeflator inflation device and disposable three-way tap (manifold) were considered semi-critical items [17,18].
RESULTS
In this study, 60 patients submitted to angioplasty were accompanied. Twenty-six of the patients presented with adverse events. In these patients 29 new and 176 recycled medical products were utilized. Of the total, 22 patients presented one or two adverse events and four patients three or four events.
There was no significant difference in the utilization of recycled products between the groups of patients who presented with adverse clinical events compared to those without events.
The occurrence of hypotension in 11 (18.3%) patients also did not give a statistically significant difference (p-value = 0.91), that is, it was not associated to the reuse of medical products.
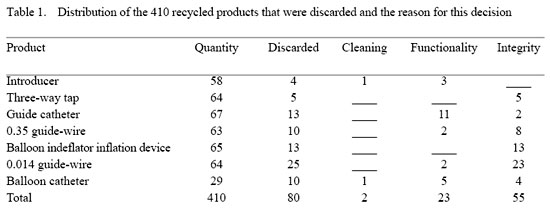
In the 60 patients submitted to angioplasty, 486 medical products were employed; 76 for the first time and 410 recycled. Four (5.63%) medical products used for the first time were discarded, (1 introducer sheaf, two three-way taps, one 0.014 guide wire) three (75%) of which were discarded as they were incomplete (Table 1).
Of the 410 recycled products utilized in the 60 patients submitted to angioplasty, 80 (19.4%) were discarded before (on preparing the surgical table) or during the procedures. Of these 18 (28.1%) 0.014 guide wires were discarded before the procedure and seven (10.9%) were discarded during the procedure.
Fifty-five products were discarded as they were incomplete with all the indeflator inflation devices discarded for this reason. Two products were rejected as they were not clean (one introductory sheaf and one balloon catheter). Eleven guide catheters were discarded because of resistance during insertion at the time of the procedure.
DISCUSSION
Adverse clinical events are recognized as one of the greatest problems in healthcare [10] and monitoring this is necessary particularly in respect to the reuse of medical products. With cardiac procedures, several studies have been done with the objective of describing the association of adverse events with the recycling of medical products [19-21].
In the current study, hypotension occurred in 13 (24%) of the 60 patients. This was the most common adverse event. Jacobson et al. [22] in 1983 described similar results in 341 patients submitted to coronary angioplasty using brand new and recycled medical products; the overall occurrence of hypotension was 27% but this was not associated with the reuse of equipment.
Frank et al. [23] in 1988 performed a prospective study over the period of November 1986 to June 1987 involving 414 patients submitted to angioplasty using brand new and recycled medical products. The patients were divided into three groups. In the first group, formed by 161 patients, 426 disposable catheters were employed, the second of 152 patients used 384 recycled catheters that had been reprocessed a maximum of two times. Finally, group three was formed of 101 patients who utilized 325 catheters that had been reprocessed more than two times. The temperature of the patients after the procedures did not pass 38.2ºC (37.4ºC in 25 - 6% - of the patients). The differences among the three groups in respect to fever were not significant. Infectious complications associated with catheterization did not occur in any of the cases. Of the 414 patients, 38 (9.2%) developed hematomas at the entry site. In the current study, five (11%) patients presented with bleeding at the point of entry. No episode of fever or infection was seen.
In Canada, Plante et al. [20] performed a prospective observational two-centered study over 10 months to evaluate the cost-benefit and efficacy of balloon catheters in patients submitted to angioplasty. The study involved 694 patients (374 patients from a center that only used brand new catheters and 320 in a center that predominantly used recycled catheters) with the data reported at the end of each angioplasty. At the end of the study, the number of catheters used per lesion was higher in the center using recycled products as in some cases the balloon catheters were unable to unblock the vessels and were substituted for brand new catheters. Consequently, the time of procedure and the quantity of contrast were greater. The temperature was measured one hour before the procedures and at each eight hours after. Adverse events presented by the patients were not associated to the reuse of medical products.
Grimandi et al. [24] in 1996 made a controlled comparative experimental study evaluating the mechanical and microbiological risks of reprocessing and reuse of catheters in angioplasty compared to new catheters. Sterility, pyrogenic and mechanical analysis were performed. They concluded that the reuse of catheters in angioplasty should not be practiced as in the study they found pyrogenic substances and cellular remnants after sterilization.
Luijt et al. [25] in 2000, with the aim of determining the risk of viral transmission, performed a non-controlled experimental study involving disposable 5F cardiovascular and endovascular balloon catheters. The catheters were tested to verify the presence of viruses at four different stages: after contamination, cleaning, sterilization and after simulation of reuse. The study showed that the removal of virus from catheters is difficult. After extensive cleaning and sterilization, catheters continue to be contaminated with infectious enterovirus, although infectious adenoviruses were not detected. They concluded that, although the process of cleaning and sterilization is rigorous, the virus continues to be present on the catheters and thus reutilizing diagnostic or intervention cardiovascular and endovascular catheters is dangerous and should not be practiced.
Fagih et al. [26] in 1999 performed a non-systematic review of publications on the possible transmission of Creutzfeldt-Jakob disease with the reuse of medical products utilized in angioplasty. This review did not find any reported cases and the authors concluded that there is no scientific evidence of this transmission.
Other aspects should be studied when an institution intends to recycle medical products. Products that might be functioning defectively or for which there are doubts related to safety or performance after reprocessing need to be identified and discarded. The level of inspection depends on the complexity of the product and its function. The process of inspection may be a simple visual inspection or complex tests related to the physical integrity and functionality by the biomedical engineering section of the hospital [27].
Zubaid et al. [28] in 2001 performed a study comparing the efficiency and efficacy of brand new and recycled balloon catheters. The authors accompanied 359 patients (377 lesions) in the period from February 1999 to December 2000 involving 178 recycled and 199 brand-new catheters. When patients presented with more than one coronary lesion, only one was chosen for the study and the surgeon chose the catheter depending on the type of lesion. After each angioplasty, the balloon catheter was inspected. This study demonstrated that there was no significant difference in the efficiency of brand new and recycled balloon catheters. During the current study, five recycled balloon catheters were discarded as insufflation was inadequate.
The possibility of rupture of medical products, in particular balloon catheters, is one concern of their reuse and also fragments that break off the equipment can result in embolia or cause damage to vessel walls leading to clinical complications and prolonged procedures. Moreover, the sterilization of equipment used in angioplasties with ethylene oxide may change the physical characteristics of the polymer and change the physical integrity of catheters [1]. In the current study, four balloon catheters, 23 0.014 guide-wires and 13 balloon indeflator inflation device were discarded for being incomplete.
Chou et al. [29] in 2002 reported two cases of patients submitted to angioplasty using recycled balloon catheters resulting in the rupture of the balloons after insufflation. They reported that in 5000 cases, only these two cases of rupture occurred.
Diagnostic catheters can also present ruptures after about 10 reuses however there are no in vivo studies comparing the performance of new with recycled diagnostic catheters. Schneider et al. [21] in 1983 found apparent aging of polyurethane diagnostic catheters and the stainless steel of guide wires.
Other alterations presented by medical products are changes in physical characteristics that do not allow their functionality. In the current study, the guide-wires that were discarded presented resistance to enter the introducer sheaf.
Cleaning is the most important stage of recycling medical products as, when not adequate, there is a possibility that disinfection and sterilization will not be efficient [2]. Two recycled medical products, one introducer sheaf and one balloon catheter, were discarded as they were dirty.
On defining the number of times that each product can be reprocessed, it is necessary to guarantee a control for each team that uses the product, as each of these products is composed of different polymers which behave in different manners at each stage of reprocessing [30].
The medical article reutilization commission of a hospital should continually evaluate if the recycling protocol and the quality control of recycled products are efficacious to guarantee the safety of the patient and that the cost-benefit fulfills the proposed objectives [31].
CONCLUSION
There is no association between the reuse of medical products in coronary transluminal angioplasties and the occurrence of adverse clinical events, suggesting the possibility of recycling as long as rigid quality control protocols are adopted.
REFERENCES
1. Mark K-H, Eisenberg MJ, Eccleston DS, Cornhill JF, Topol EJ. Reuse of coronary angioplasty equipment: technical and clinical issues. Am Heart J. 1996;131(3):624-30.
2. Graziano KU, Castro MES, Moura MLPA. A importância do procedimento de limpeza nos processos de desinfecção e esterilização de artigos. Rev SOBECC. 2002;7(3):9-23.
3. Instituto do PVC. O PVC. [citado 2000 out. 22]. Disponível em: URL: http://www.institutodopvc.org/pvc.htm
4. Reichert M, Young JH. Sterilization technology for the health care facility. 2ª ed. Maryland:Aspen;1997.
5. Diretrizes da Sociedade Brasileira de Cardiologia sobre angioplastia transluminal coronária. Arq Bras Cardiol. 1995;64(5):491-500.
6. Gottschall CAM. Angioplastia coronária. In: Nesralla I, ed. Cardiologia cirúrgica: perspectiva para o ano 2000. 5ª ed. São Paulo:BYK;1994. p.499-523.
7. Hudak CM, Gallo BM. Modalidades de tratamento: sistema cardiovascular. In: Hudak CM, Gallo BM, eds. Cuidados intensivos de enfermagem: uma abordagem holística. Rio de Janeiro:Guanabara Koogan;1997. v.1. p.194-282.
8. Hammond KA. Avaliação dietética e clínica. In: Mahan K, Escott-Stump S, eds. Krause: alimentos, nutrição & dietoterapia. 10ª ed. São Paulo:Roca;2002. p.341-66.
9. Obesity: prevent and managing the global epidemic: report of WHO consultation on obesity, Geneva, 1997 jun. 3-5. Geneva: World Health Organization;1998.
10. Gallotti RMD. Eventos adversos: o que são? Rev Assoc Med Bras. 2004;50(2):114.
11. Lambertuci JR, Nunes F, Rayes A. Febre. In: López M, Laurentys-Medeiros J, eds. Semiologia médica: as bases do diagnóstico clínico. 4ª ed. Rio de Janeiro:Revinter; 2001. v. 1. p.83-99.
12. Lopez M. Pressão arterial. In: Porto CC, ed. Semiologia médica. 4ªed. Rio de Janeiro:Guanabara Koogan;2001. p.315-47.
13. Porto CC, Rassi S, Silva FP. Pressão arterial. In: Porto CC, ed. Semiologia médica. 4ªed. Rio de Janeiro: Guanabara Koogan; 2001. p.466-77.
14. Ministério da Saúde. Reunião de peritos para a normalização do uso e reutilização de materiais médico-hospitalares descartáveis no país. Brasília (DF): Centro de documentação;1985. [Série D: reuniões e conferências nº 4].
15. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Portaria nº 4, de 7 de fevereiro de 1986. Normatiza o uso e reutilização de materiais médico-hospitalares descartáveis no país. Diário Oficial da União, Brasília (DF), 12 fev. 1986, sec.1, p. 2327.
16. Ministério da Saúde. Processamento de artigos e superfícies em estabelecimento de saúde. 2ª ed. Brasília (DF): Coordenação de Controle de Infecção Hospitalar;1994.
17. Associação Paulista de Estudos e Controle de Infecção Hospitalar (APECIH). Esterilização de artigos, em unidade de saúde. São Paulo;1998.
18. Rutala WA. Desinfection, sterilization and wast disposal. In: Wenzel RP, ed. Prevention and control of nosocomial infection. Baltimore:Willian & Wilkins;1993. p.460-96.
19. Cookson ST, Nora Jr JJ, Kithas JA, Arduino MJ, Bond WW, Miller PH et al. Pyrogenic reactions in patients undergoing cardiac catheterization associated with contaminated glass medicine cups. Cathet Cardiovasc Diagn. 1997;42(1):12-8.
20. Plante S, Strauss BH, Goulet G, Watson RK, Chisholm RJ. Reuse of balloon catheters for coronary angioplasty: a potential cost-saving strategy? J Am Coll Cardiol. 1994;24(6):1475-81.
21. Schneider RM, Fornes RE, Stuckey WC, Gilbert RD, Peter RH. Fracture of a polyurethane cardiac catheter in the aortic arch: a complication related to polymer aging. Cathet Cardiovasc Diagn. 1983;9(2):197-207.
22. Jacobson JA, Schwartz CE, Marshall HW, Conti M, Burke JP. Fever, chills and hypotension following cardiac catheterization with single-and multiple-use disposable catheters. Cathet Cardiovasc Diagn. 1983;9(1):39-46.
23. Frank U, Herz L, Daschner FD. Infection risk of cardiac catheterization and arterial angiography with single and multiple use disposable catheters. Clin Cardiol. 1988;11(11):785-7.
24. Grimandi G, Sellal O, Grimandi F, Crochet D. Risks of reusing coronary angioplasty catheters: results of an experimental study. Cathet Cardiovasc Diagn. 1996;38(2):123-32.
25. Luijt DS, Schirm J, Savelkoul PH, Hoesktra A. Risk of infection by reprocessed and resterilized virus-contaminated catheters: an in-vitro study. Eur Heart J. 2001;22(5):378-84.
26. Fagih B, Eisenberg MJ. Reuse of angioplasty catheters and risk of Creutzfeldt-Jakob disease. Am Heart J. 1999;137(6):1173-8.
27. Reuse of single-use medical devices: making informed decisions. Philadelphia: ECRI;1996.
28. Zubaid M, Thomas CS, Salman H, Al-Rashadan I, Hayat N, Habashi A et al. A randomized study of the safety and efficacy of reused angioplasty balloon catheters. Indian Heart J. 2001;53(2):167-71.
29. Chou M-T, Cheng B-C, Huang T-Y. Catheter disconnection: a potential risk of reused coronary angioplasty catheters. Acta Cardiol Sin. 2002;18:88-92.
30. Reuse of cardiology catheters. An application to the Committee on Reuse of Disposable Patient Care Products. The division of cardiology- Royal Alexandra Hospital. Edmonton, Canada; Sep. 1992.
31. Baffi SHO. Reprocessamento e reutilização de cateteres de hemodinâmica: a busca da qualidade nesta prática [Dissertação]. São Paulo: Universidade de São Paulo; 2001.
 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license