The change in the heparin solution trade mark in Brazil that had been commonly used in cardiac surgery has shown increased number in the coagulopathy, re-exploration and other side effects in our Institution and others.
All four different heparin solutions available in the Brazilian market were studied in the Connective Tissue Lab, HUCFF, UFRJ and compared to the Liquemine (out of the market) and the international control solution. All samples were evaluated by magnetic nuclear resonance as well as their anticoagulant effectiveness.
There were significant differences among them regarding the anticoagulant activity. It was also observed contamination with other dermatan sulfate, samples chemically degraded and with significant change in the molecular weight.
Among the studied samples, none of them can offer security in cardiac surgeries on pump. None of them has demonstrated similar quality to Liquemine, which is not available in the Brazilian market.
A mudança na marca da heparina rotineiramente utilizada nas cirurgias cardíacas no Brasil tem sido acompanhada por aumento do número de casos de discrasia sanguínea, aumento de reoperações e efeitos adversos em nossa Instituição e em outras.
Foram avaliadas no Laboratório de Tecido Conjuntivo do HUCFF/UFRJ, quatro preparações disponíveis e comparadas à heparina retirada do mercado (Liquemine) e ao padrão de controle internacional. As preparações de heparina foram submetidas à ressonância nuclear magnética para avaliação da integridade estrutural, bem como avaliação de sua eficácia anticoagulante.
Houve diferença significativa quanto à atividade anticoagulante entre as amostras. Também se observou a presença de contaminação com dermatam sulfato, amostras degradadas quimicamente e com significativa alteração do peso molecular.
Das amostras estudadas, nenhuma atendeu aos requisitos de segurança para utilização em cirurgias cardíacas com circulação extracorpórea. Nenhuma delas apresentou a qualidade semelhante ao Liquemine, não mais disponível no mercado brasileiro.
INTRODUCTION
Heparin is a sulfated polysaccharide and has been used for more than 50 years as an anticoagulant agent [1]. This polymer, obtained from animal tissues (either from porcine intestinal mucosa or, rarely, from bovine lungs), is the second most used natural therapeutic agent in the world, exceeded only by insulin [2]. Thanks to the discovery of heparin, we have seen one of the most important advances in the development of cardiac surgeries using cardiopulmonary bypass (CPB).
This anticoagulant, routinely used during CPB, has the advantage of being very specific, of not producing anaphylaxis allergies. Thrombocytopenia is the most significant side effect, but it is rarely fatal, and can occur only after a relatively prolonged use of 5 to 7 days.
Non-fractionated heparin contains a mixture of polysaccharides with molecular weight between 3.000 and 30.000 Daltons. It acts in the final stage of coagulation cascade and concomitantly activates antithrombin and heparin cofactor II. It inhibits thrombin and factor Xa, and impedes the conversion of fibrinogen into fibrin. The heparin increases the inactivation of thrombin up to 2.000 times [3]. Its antidote is protamine, which is widely used in cardiovascular surgery.
The systemic anticoagulation for CPB is obtained with an initial dose of heparin of 3 to 4 mg for every kilogram of weight or 300-400UI/kg and is maintained by administering additional doses of 0.5 to 1 mg for every kilogram of weight during each hour of perfusion. It has been accepted by most services that an appropriate anticoagulation during CPB should increase the coagulation time by 3 to 4 times its baseline value. Thus, activated coagulation time (ACT) would have a value equal or greater than 480 seconds.
Until now, all heparin produced is still obtained from porcine intestinal mucosa, leading to a growing shortage of raw materials in the international market. Worldwidely, it is estimated that, to treat about 20 million victims of thromboembolism, the mucosa of more than 200 million pigs are needed to meet this yearly demand for heparin. Furthermore, the launch of low molecular weight heparin increased the annual consumption of the agents by 10 to 20% [4].
Different commercial preparations of heparin are available in the Brazilian market. However, most of these preparations require a detailed analysis of the product's purity, structural integrity and anticoagulant power. Brazilian standards require that the control of nonfractionated heparin preparations must be exclusively based on a test of the coagulation time of citrated plasma. This method is currently not in use [5].
Recently, the removal of heparin preparation by the Roche Laboratory in Brazil has coincided with higher rates of reoperations due to bleeding and postoperative blood dyscrasia in both our Institution and others. The aim of this study was to evaluate the quality of heparin preparations available in the Brazilian market, as well as those that are currently used in cardiovascular surgeries with cardiopulmonary bypass.
METHODS
All heparins were systematically submitted to the tests described below. The commercial origin of the preparations 2 to 5 has not been identified. Only preparation 1 could be identified as Liquemine, produced by Roche, which is no longer available on the market and which is used as a reference in some tests.
1. Activated Partial Thromboplastin Time (APTT)
Human plasma was collected in a 3.8% sodium citrate solution at a ratio of 9:1 and examined by Activated Partial Thromboplastin Time (APTT). In short, a "pool" of 90 ìL of normal plasma was incubated for 1 minute (min.) at 37°C with 10 ìL of the heparin solutions in different concentrations. After 1 minute, 100 ´L of activated cefalin (Wiener Lab) was added to the mixture, and it was incubated for another 2 minutes. Then, 100 ìL of CaCl
2 0.025 M was added, and the coagulation time was recorded in a microcoagulometer (Amelung, Germany), model KC4A.
2. Nuclear Magnetic Resonance (NMR)
The NMR spectra were obtained using a Bruker XRD 600 machine with a triple resonance probe. After dialysis and lyophilization, about 10 mg of different heparins were dissolved in 0.5 mL of D
2O 99.9% (Cambridge Isotope Laboratory) and the analyses were performed at 600 MHz at 60°C with water suppression via presaturation.
3. Gel Filtration chromatography
Gel Filtration Chromatography was performed using a Sephacryl S400 HR column that was 1.5 cm in diameter and 200 cm height. The column was pre-balanced with 0.2 M ammonium bicarbonate buffer (pH 7.0 for 24 h). Ampoules of Liquemine (Roche) and from Preparation 2 containing 5 mL of solution 5000 IU/mL were then applied in this column and eluted with the same buffer using a flow of 18.0 mL/min. Samples of 3.0 mL were collected and analyzed by their metachromatic properties, measuring the absorbance at 525 nm on a spectrophotometer (Ultraspec 3100 pro - Amersham Biosciences) in the presence of 1.9 dimethylmethylene blue.
4. Dosage of hexuronic acid
The dosage of hexuronic acid was administered using the carbazole method, using 200 ìL as sample and as glucuronolactone as a standard. Initially, 1.0 ml of 0.9% sodium tetraborate (Merck) was combined with 98% sulfuric acid (Merck) upon the sample. This mixture was homogenized and kept at 100°C for 12 minutes. Then the tubes were cooled on an ice bath for 5 minutes, and 40 ìL of a 0.2% carbazol solution (Merk) was then combined with 100% ethanol (Merck). The tubes were homogenized again and maintained at 100°C for 10 minutes. Finally, the samples were cooled on the ice bath for 5 minutes and then homogenized, and absorbance was measured at 525 nm on a spectrophotometer (Ultraspec 3100 pro - Amersham Biosciences).
RESULTS
Table 1 and Figure 1 show that commercial preparations of heparin available in Brazil have significant differences in their anticoagulant activity. In some cases, we noted only 70% of the declared amount (in terms of anticoagulant activity). Because of this, we note a larger amount of heparin in some preparations. The aim is to attempt to obtain the same anticoagulant activity that those preparations considered ideal; that is, we found that the heparin in Preparations 4 and 5 present a specific activity (IU/mg), which is significantly lower than that observed in Preparation 1 (Liquemine).
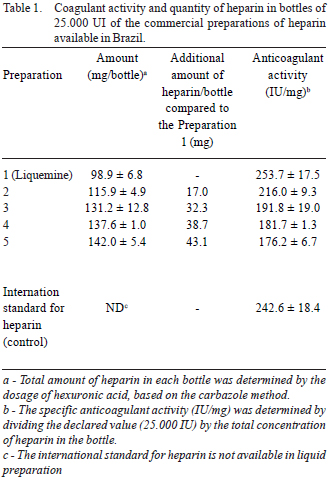
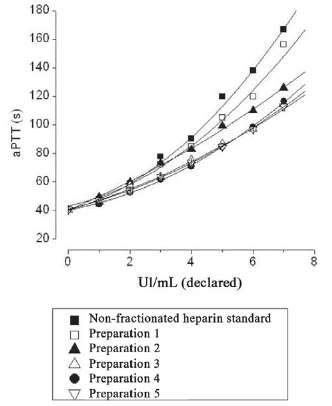
Fig. 1 - Comparison of anticoagulant activity by APTT, in seconds, from various preparations of analyzed heparin to consider the quantity (UI) declared on bottles by their manufacturers. Note the proximity of the curve between the international standard and Preparation 1, considered appropriate for surgery with cardiopulmonary bypass
The structural integrity of heparin in each Preparation was measured on the NMR spectra. This methodology allows us to clearly identify signs of anomeric protons (H5 of iduronic acid and the protons of the N-acetyl group of heparin). These protons are present in the structure of heparin, shown in Figure 2. We note significant structural changes in samples of non-fractionated heparin available in the Brazilian market (Figure 3). About 30% of glucosamine residue is 6-O disulfated in some samples (Preparations 4 and 5; Figures 3E and 3F). This structural change results in a significant reduction in heparin's anticoagulant activity (Table 1).
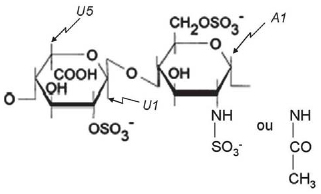
Fig. 2 - Structure of heparin. Heparin is mostly composed of alternating units of iduronic acid 2-sulfate and N- and 6-O disulfated glucosamine. Some residues of glucosamine are N-acetylated. The protons that are easily identifiable on the NMR unidimensional spectrum are indicated in the panel: U1 and A1 are the anomeric protons of iduronic acid and glucosamine, respectively; U5 corresponds to the H5 of iduronic acid
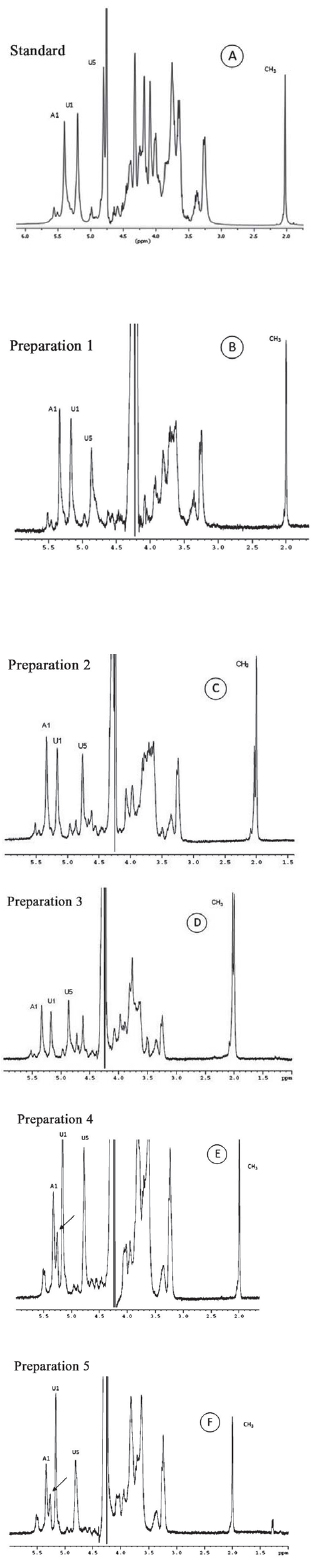
Fig. 3 - Unidimensional spectrum of proton from the international standard of heparin (A) and commercial preparations available in Brazil (B-F). Note the similarity between the spectra of standard heparin (A) and Preparation 1, Liquemine (B). Preparations 2-3 (C and D) contain an additional sign of CH3, at approximately 2.00 ppm, due to contamination from dermatam sulfate. Preparations 4 and 5 (E and F) contain an additional signal of anomeric protons, indicated by the arrow on the panel. Detailed study of these preparations of heparin using two-dimensional spectra of proton showed that this additional signal was due to the presence 6-O disulfated glucosamine residue
Preparations 2 and 3 do not present structural alteration; however, they are probably contaminated with dermatam sulfate, another type of glycosaminoglycan that is also present in porcine intestines (Figures 3C and 3D). The presence of this contaminant reveals without a doubt that the heparin was not appropriately purified, even though the compound apparently did not present toxic effects.
The reports from various cardiovascular surgery services in the country that used Preparations 2 and 3 were unfavorable due to the amount of bleeding, although a quantitative evaluation is necessary.
Among the laboratory analyses from the Connective Tissue Laboratory of HUCFF - UFRJ, we did not find any preparations of non-fractionated heparin contaminated with oversulfated condroitin sulfate as described for some samples from the United States and European countries [6].
The molecular weight of Preparations 1 (Liquemine) and 2 was compared using Sephacryl S400 gel filtration (Figure 4). Preparation 2 clearly presents a component with lower molecular weight than Preparation 1, which corresponds to approximately 20% of the mixture.
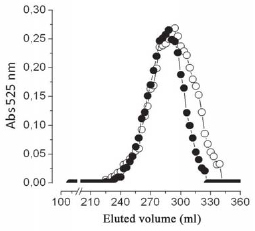
Fig. 4 - Comparative analysis of the molecular weight of the preparations 1 (n) and 2 (O), using Sephacryl S400 HR gel filtration
Traditionally, the heparin made available in Brazil from the Roche company (Liquemine, identified as Preparation 1 in this study) was almost universally used in cardiovascular surgeries with cardiopulmonary bypass. However, the removal of this preparation from the market has created a critical situation, leading to reports from various Services of an increase of postoperative bleeding and consequent reoperations.
The aim of this study was to determine which preparations (among those that remain available in the Brazilian market) contain the non-fractionated heparin that is most appropriate for operations with cardiopulmonary bypass.
Clearly, Preparations 4 and 5 of heparin are not appropriate because they contain chemically degraded heparin (indicated by an additional signal of anomeric protons on the NMR spectrum between A1 and U1, Figures 3E and 3F), and present significantly reduced anticoagulant activity. Preparations 2 and 3 do not present structural changes, but present contamination from dermatam sulfate (a compound that likely does not cause toxic effects, but that reflects low quality control).
The reports from cardiovascular surgery services using these two preparations of heparin (2 and 3) were unfavorable, although a more significant quantitative evaluation may be necessary.
Our results showed a significantly greater amount of heparin in bottles of Preparations 2 to 5 when compared to the Preparation 1 (Liquemine), reinforcing the fact that these preparations have a specific activity (in IU/mg) that is significantly lower than Preparation 1. This reduced anticoagulant activity may be responsible for consumption coagulopathy during use of CPB, resulting in a clinical presentation of blood dyscrasia.
These results raise another intriguing question: Why do non-fractionated heparins without significant structural differences, such as Preparations 1 and 2, have different specific anticoagulant activity (in IU/mg)?
Answering of this question could explain the differences in side effects noted between the Preparations of nonfractionated heparin. The possible answer is shown in the Figure 4. In this experiment, we compared the molecular weight of non-fractionated heparins found in Preparations 1 (Liquemine) and 2. Preparation 2 has a lower component of molecular weight when compared to the Preparation 1, indicated by the shaded area in Figure 4. This fraction corresponds to about 20% of the total sample. Coincidentally, this amount of heparin is the same amount that was added to Preparation 2 (compared to the Preparation 1) to ensure the anticoagulant activity declared on the bottle (Table 1).
The explanation for this result is that the fraction of lower molecular weight does not meet the coagulation test, especially the APTT. As a result, this non-fractionated heparin is not neutralized by protamine at the end of cardiopulmonary bypass, since it is not detected by the APTT test and remains in circulation. Therefore, it can cause pre- and post-operative bleeding.
In order to use non-fractionated heparin safely in patients during procedures that are dependent on cardiopulmonary bypass, our results suggest that physicians must be provided with preparations with guaranteed chemical purity, free of contaminants and with high specific anticoagulant activity.
CONCLUSIONS
The world market for heparin is currently a complex system. The increasingly strict analyses of preparations of non-fractionated heparin (mainly in the United States and Europe) may motivate manufacturers from eastern countries to send their lower-quality preparations to countries that accept less rigorous analyses of these molecules.
Recently, other countries have reported adverse events observed in the use of heparin, which were attributed to contamination by an oversulfated condroitin sulfate [6].
We lack specific regulations for the analysis of preparations of heparin using modern and appropriate methods. Suppliers from the domestic market also have little interest in in controlling the quality of non-fractionated heparin. Moreover, we need the heparins used in hospitals to undergo careful clinical evaluations by means of quantitative criteria.
None of the evaluated samples met the necessary requirements of the aforementioned proposition; they all fared poorly in terms of chemical purity, absence of contaminants and high specific anticoagulant activity, unlike Preparation 1 (Liquemine).
REFERENCES
1. Croti UA. Cartas ao editor. Rev Bras Cir Cardiovasc 2003; 18(4): 378.
2. Pinto Jr. VC, Daher CV, Sallum FS, Jatene MB, Croti UA, Situação das cirurgias cardíacas congênitas no Brasil. Rev Bras Cir Cardiovasc 2004; 19(2): III-IV.
1. Crafoord C, Jorpes E. Heparin as a prophylactic against thrombosis. JAMA. 1941;116:2831-5.
2. Jin L, Abrahams JP, Skinner R, Petitou M, Pike R, Carrel RW. The anticoagulant activation of antithrombin by heparin. Proc Natl Acad Sci USA. 1997;94(26):14683-8.
3. Ofuso FA, Gray E. Mechanisms of action of heparin: applications to the development of derivatives of heparin and heparinoids with antithrombotic properties. Semin Thromb Hemost. 1988;14(1):9-17. [
MedLine]
4. Alban S. The 'precautionary principle' as a guide for future drug development. Eur J Clin Invest. 2005;35(Suppl 1):33-44. [
MedLine]
5. Hirsh J, Raschke R. Heparin and low-molecular-weight heparin: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):188S-203S. [
MedLine]
6. Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, et al. Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med. 2008;358:(23):2457-67. [
MedLine]
This study was carried out with the support of FAPERJ - Foundation for Research Support of the State of Rio de Janeiro, Ministry of Health and National Counsel of Technological and Scientific Development (CNPq).





 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license