Andrés Di Leoni FerrariI; Anibal Pires BorgesI; Luciano Cabral AlbuquerqueII; Carolina Pelzer SussenbachI; Priscila Raupp da RosaI; Ricardo Medeiros PiantáI; Mario WieheII; Marco Antônio GoldaniI
DOI: 10.5935/1678-9741.20140104
RESUMO
A estimulação cardíaca artificial (ECA) é o tratamento mais seguro e eficaz para a bradicardia sintomática irreversível. Nas indicações propícias, pode trazer grande benefício clínico. Contudo, as evidências mostram que a ação da ECA geraria, em alguns casos, efeitos deletérios à estrutura e fisiologia cardíacas. Este potencial efeito negativo da ECA convencional poderia ser mais acentuado principalmente em pacientes com comprometimento prévio da função ventricular esquerda e, sobretudo, quando o eletrodo é colocado em posição apical do ventrículo direito (VD). Intrigante é, contudo, que após quase 6 décadas de ECA do VD, apenas uma reduzida parcela de pacientes apresenta esta condição clinicamente manifesta. Os determinantes do surgimento ou não da cardiopatia por ECA não estão totalmente esclarecidos. Ainda é motivo de debate a existência de uma relação de causalidade entre o padrão de BRE artificial secundário à ativação antifisiológica ventricular, alterações da dinâmica contrátil ventricular, e condições clínicas (disfunção sistólica prévia, cardiopatia estrutural preexistente, tempo desde o implante) ou elétricas (duração do intervalo QRS, dose percentual de estimulação ventricular). Esta revisão aborda dados contemporâneos sobre esta nova entidade e discute alternativas de como utilizar a ECA neste contexto, com ênfase na terapia de ressincronização cardíaca.
ABSTRACT
Implantable cardiac pacing systems are a safe and effective treatment for symptomatic irreversible bradycardia. Under the proper indications, cardiac pacing might bring significant clinical benefit. Evidences from literature state that the action of the artificial pacing system, mainly when the ventricular lead is located at the apex of the right ventricle, produces negative effects to cardiac structure (remodeling, dilatation) and function (dissinchrony). Patients with previously compromised left ventricular function would benefit the least with conventional right ventricle apical pacing, and are exposed to the risk of developing higher incidence of morbidity and mortality for heart failure. However, after almost 6 decades of cardiac pacing, just a reduced portion of patients in general would develop these alterations. In this context, there are not completely clear some issues related to cardiac pacing and the development of this cardiomyopathy. Causality relationships among QRS widening with a left bundle branch block morphology, contractility alterations within the left ventricle, and certain substrates or clinical (previous systolic dysfunction, structural heart disease, time from implant) or electrical conditions (QRS duration, percentage of ventricular stimulation) are still subjecte of debate. This review analyses contemporary data regarding this new entity, and discusses alternatives of how to use cardiac pacing in this context, emphasizing cardiac resynchronization therapy.
%CVP: Percentual ventricular pacing
AAI: Atrial detection and stimulation
ACP: Artificial cardiac pacing
AF: Atrial fibrillation
AV: Atrioventricular
AVB: Atrioventricular block
HF: Heart failure
HR: Heart rate
iAV: Atrioventricular interval
ICD: Implantable cardioverter-defibrillators
LBBB: Left Bundle Branch Block
PPM: Permanent pacemaker
RMit: Mitral regurgitation
SND: Sinus node dysfunctions
VVI: Ventricular detection and pacing
INTRODUCTION
Artificial cardiac pacing (ACP) is a consolidated, safe and effective treatment strategy that has been evolving for 60 years. A large number of devices are implanted annually in Brazil and the world. Although permanent pacemakers (PPM) and implantable cardioverter-defibrillators (ICD), associated or not with cardiac resynchronization therapy (CRT), have improved the prognosis of millions of patients[1], in some cases, cardiomyopathy appears as a result of the artificial anti-physiological ventricular activation induced by the ACP. The goal of this review is to address the evidence on the causes and consequences related to this secondary heart disease in relation to the profile of patients where this condition is more prevalent, in addition to the possible strategies to prevent it from occurring in patients who need ACP.
Artificial cardiac pacing in different contexts
The classic recommendations for the implantation of electronic ACP devices have been properly listed in the various Guidelines on the subject2-5]. In general, PPMs are associated with an improvement in symptoms and quality of life in sinus node dysfunctions (SND), but without any significant impact on survival[2-5]. The prescribed systems are dual chamber, with its variations (DDD - sequential atrioventricular detection and stimulation; DDI - detection and atrioventricular pacing not synchronized to the P wave), or the single-atrial chamber system (AAI - atrial detection and stimulation). The latter, only in well-selected patients, when the atrioventricular (AV) conduction has been preserved. The single ventricular chamber mode (VVI - ventricular detection and pacing) would not bring any benefits because of the worse clinical outcome (loss of AV synchrony during sinus rhythm with risk of appearance of the pacemaker syndrome)[2,6]. When the heart rate (HR) response is inadequate for the patient's level of activity (chronotropic incompetence), and when it has to be brought towards the metabolic demand of the moment, the enabling of biosensors is indicated. In this case, the letter R is added to the nomenclature, which indicates the mode of operation of the device. For example: DDD-R.
In patients with symptomatic atrioventricular block (AVB), the dual chamber mode is once again the one of choice, especially because it maintains the AV sequence, although in specific patient profiles, as well as in chronic atrial fibrillation (AF) with slow ventricular response, VVI pacing offers similar results[7,8]. Less often, in 1st degree AVB with a very prolonged PR interval (PRi) (>300 ms), dual chamber ACP is employed to correct the dyssynchrony due to the AV decoupling[2-5,9-11].
Atrioventricular, intraventricular and interventricular synchrony
In the 1990s, Pachón et al.[6] described the ventricular pacemaker syndrome. They observed that right ventricular ACP produced negative effects on the myocardial structure and function, with clinical expression of heart failure (HF). These harmful effects originated primarily in ventricular dyssinchrony (QRS widening), artificial left bundle branch block pattern (LBBB), the appearance or worsening of mitral regurgitation (RMit), and other effects attributed to the action of the anti-physiological activation of the RV (Chart 1). They distinguished it from classic pacemaker syndrome, which is typically caused by AV dyssynchrony in patients with PPM[6].

It is known that maximum cardiac efficiency depends on the mechanically synchronous contraction of the ventricular walls, enabled by the coordinated action of different segments through the propagation of the electrical stimulus in all components of the conduction system[12-15]. This is represented graphically in the ECG by duration of the QRS interval (diQRS) of less than 120ms. Conventional ACP of the RV alters the morphology and the diQRS, varying according to the degree of activation of the specific conduction tissue, the presence of previous heart disease and the topography of the electrode in the RV.
The appearance of abnormal electrical delay because of the spreading of the stimulus outside the physiological conduction system, compromises the mechanical ventricular efficiency and characterizes the dyssynchrony. It is generally acknowledged[6,16] that the LBBB pattern alters the contractile pattern of the ventricular walls and promotes the paradoxical contraction of the interventricular septum. It also causes dyssynchrony of the papillary muscles, with additional functional impairment of the RMit[17], generating loss of systolic efficiency and higher morbidity and mortality due to HF[6,10,12-15,18-23]. It is estimated that up to 50% of the cases of ACP of the RV evolve in this way, and this would explain the reason why some patients do not present a satisfactory clinical improvement, even though the electrical disturbance is being treated by the ACP[6].
The myocardial contraction by the ACP would be hemodynamically efficient, but mechanically anti-physiological. This forces the patient to live with a certain degree of ventricular dysfunction and dyssynchronopathy, which is clinically more or less evident depending mostly on the myocardial functional reserve mechanisms and on the pre-existing myocardial condition[18]. For this reason, in some cases of normal hearts, LBBB induced by ACP may apparently not cause a very significant impairment of the left ventricular function, or the dysfunction may remain subclinical and take years to be noticed, making it seem that other factors were involved in this dyssynchronopathy[24-27].
Hori et al.[27], on the other hand, suggested that electromechanical dyssynchrony in the context of ACP could be more serious if associated with systolic dysfunction and LBBB from other causes, more than when normal LV ejection fraction (LVEF) and LBBB by artificial activation of the RV occurs isolated. However, the diQRS could not exactly reflect the degree of mechanical dyssynchrony generated by the ACP in patients with preserved systolic function. Although the ECG appears similar, LBBB from ACP has some peculiar characteristics[28]. When analyzed by Tissue Doppler Echocardiography, the artificially induced contractile delay pattern can be different when compared to LBBB from other causes, not necessarily expressing a significant underlying heart disease[29]. The BioPace study[30] (follow-up longer than 5 years of more than a thousand patients) is under way, which addresses the potential secondary negative effects of ACP of the RV prospectively, especially whether the electrical dyssynchrony generated by artificial LBBB is comparable to the LBBB of other etiologies, or if the damage depends on the pre-existence of ventricular dysfunction or structural heart disease.
Still from a hemodynamic perspective, it is said that the maintenance of intra- and interventricular synchrony would be more important than the AV synchrony for a better myocardial function[6,11]. These events, however, are definitely related. When we program the atrioventricular interval (iAV), and depending on the underlying disease (SND or AVB), we can generate different percentage ("doses") of cumulative ventricular pacing (%CVP) and, therefore, potentially several degrees of dyssynchronopathy[20,21], which may already be present with a CVP between 25% and 40%[5,31,32]. Despite the great theoretical importance of the sequential atrioventricular electrical coordination, this does not mean the certainty of mechanical synchrony between these chambers. Studies[33,34] have found that ACP, especially when RV apical, impairs the left atrial drain, which harms ventricular filling despite maintaining the AV sequence. This effect would also be more pronounced in patients with previous systolic dysfunction, and less when the iAV is optimized and the LVEF is preserved. This stresses both, the role of better iAV to mitigate the iatrogenic potential of the artificial activation of the RV, as the importance of the existence of a substrate, for example the systolic dysfunction, for the synergism of these effects.
Evidence, myths and truths: how much is important?
The specific profile of patients predisposed or vulnerable to the emergence of harmful electromechanical effects produced by ACP is still up to debate and the subject of research. Due to factors that haven't yet been fully understood, but that are evident after 6 decades of cardiac pacing, the unfavorable clinical outcome is not a condition that can be associated 100% with ACP[13,15].
A substantial number of studies associated ACP of the RV with an increased risk of HF and other outcomes (especially atrial fibrillation). Almost none of these, however, directly registered a higher mortality, and many were based on the analysis of small populations (Table 1). Several of them are also the result of comparisons between the AAI and DDD modes against the VVI mode, counting, in the latter case, with the potential bias of AV dyssynchrony in the genesis of these alterations.
The importance of such clinical characteristics as the substrate for the development of heart disease in the context of ACP, and not necessarily because of ACP, has been emphasized in other publications. Studies show that the greatest risk of an unfavorable outcome could be determined by the pre-existence of other factors, such as: structural heart disease, systolic dysfunction (LVEF<40 %), left ventricular mass index >130g/m2 or coronary artery disease[27,32].
In patients with recommendation for PPM in SND, the risk of developing chronic AF and cerebral vascular accident (CVA) - stroke - was more strongly determined by previously present clinical variables than by artificial pacing of the RV, when AV synchrony was maintained[33-36]. A post-hoc analysis of DANPACE[37] revealed that it can be safe to use the DDD-R mode in patients with SND and without heart disease with preserved systolic function. The DDD-R pacing in this study did not generate differences in terms of HF when compared to the AAI-R mode (with intrinsic ventricular activation). After a mean follow-up of 5.4 years (which some consider insufficient for the profile of the patients under study), the absolute drop in LVEF was less than 5%. The authors stressed, however, that the clinical characteristics (age, hypertension, coronary artery disease, left ventricular diastolic diameter and/or previous systolic dysfunction) are probably the major determinants of the risk of developing symptomatic HF in the presence of ACP, independent of the site of the electrode (apical vs. non apical) and the percentage of CVP[37].
The classic MOST study[38], which was designed to demonstrate the differences between the ACP with physiological AV sequence (DDD-R) versus VVI-R in 2,010 patients with SND and preserved LVEF, revealed that a percentage of CVP exceeding 40% is an independent predictor of the onset of HF. This point is taken as a reference in several Guidelines[3-5]. Although seemingly contradictory, however, until today no full light has been shed on the reasons that explain this study's observation that in the bicameral mode, which is theoretically more physiological for the temporal preservation of AV, the required dose of CVP to trigger deleterious effects attributed to ACP corresponded to half (40% vs. 80%) of the one required in the VVIR system for the same damage[39]. A possible explanation could be the dyssynchrony aggregated to an iAV that was not ideally programmed in the DDD group, confirming that the AV sequence is, in fact, different from the concept of AV synchrony. Also relevant in this context, a sub-analysis of MOST revealed that the evolution to HF actually happens in less than 10% of cases, mostly associated with coronary artery disease or prior structural heart disease[40].
Although the literature[6,9,12,13,19,21,22] and the positioning of the scientific community are strongly indicative of the fact that any patient under the action of ACP is potentially vulnerable to the appearance of this heart disease, many of the same publications also stress that patients without previous heart disease have a good tolerance to artificial pacing of the RV[6,13,15,37]. For Sweeney and Prinzen, the risk of developing HF in 2 years in structurally normal hearts lies between 0.76% and 1.7 %, which is probably similar to what can be expected in the normal population without a PPM[13,31]. However, even depending on the relationship between the elapsed time since the implant and the percentage of CVP dose, it is interesting to see that today there are millions of patients with devices that artificially activate the RV, and we are nevertheless not experiencing an HF epidemic due to this fact.
On this point about the relationship between the time since the first implant and the progression to heart disease because of ACP in patients with congenital AVB and without known prior structural heart disease, Yu et al.[41] define heart disease associated with ACP when the LVEF <45% and there is ventricular dyssynchrony caused by artificial activation of the RV, in the absence of another cardiomyopathy that could justify it. In this profile of patients with prolonged follow-up times, an incidence of 9% in the 1st year after implantation of PPM is described[41]. Other authors presented a variable prevalence: 13% After 9.7 years (± 2.9 years) of artificial pacing of the RV[42], up to 15.4% after almost 25 years (24.6±5.6 years) of ACP[43]. When assessing the correlation between ACP, HF and the presence of specific antibodies, Sagar et al.[44] found an association with specific antibodies in a group of patients with a PPM for congenital AVB without structural heart disease. This restates that, in this context, the risk could very well not derive simply from the effect of ACP, but instead be related to other etiological agents.
As cited, several publications[15,27,45] confirm the negative effects of ACP of the RV (Table 2) in this clinical- pathological context. There is a record that the highest risk of heart disease by ACP occurs when this generates greater degrees of intraventricular dyssynchrony[43], in addition to the presence of other factors being necessary, for example, ventricular remodeling, arterial hypertension, coronary heart disease etc., reinforcing the thesis of the need for a substrate.
The vast majority of patients with implantable cardioverter-defibrillators (ICD) have a pre-existing heart disease. There are records that these conditions have a worse evolution with artificial (apical) activation of the RV, the main site for placing the shock electrode[6,18]. In these cases, the risk approximates 50% for the development of ACP's associated cardiomyopathy within 2 years[13]. Retrospective studies of the results of DAVID[32] and MADIT-II[46] indicate that ACP of the RV is less tolerated when there is previous systolic dysfunction. Up to the 1st year, HF symptoms arose in the dependence of CVP percentage (>40% in DAVID[32] and >50% in the MADIT II[46]), which was a clear dose-response relationship[22]. It is important to highlight, however, that in DAVID[32], the patients programmed for a DDD-R with a long IAV, who accumulated 11% of CVP, made up the group with the best tolerance for ACP. This could suggest that, in this population, it is best not to stimulate the RV from the apex. And if there is no other way, then it should be done with the best AV synchrony.
This hypothesis also proved to be valid according to the results of the INTRINSIC RV[47], confirming that in some scenarios, a certain CVP percentage of the RV may be necessary, provided that the AV synchrony is preserved. This study revealed after 1 year of follow-up that a high cumulative CVP percentage is associated with a higher rate of hospitalization for HF, ventricular tachycardia and mortality. However, better results were obtained in the group with a CVP between 10-19% (2.8% of occurrences) when compared to the group with <10% (8.1% of occurrences)[47]. The reason for these findings would once again lie in the inherent harm of the decoupling of the AV produced by the algorithm that minimized the activation of the RV.
It is important to highlight, however, that there is no concrete data on the role that some assisting drugs, such as those that affect the renin-angiotensin-aldosterone system and that have proven beneficial in the context of the clinical treatment of HF, would play in secondary structural remodeling of the ACP of the RV, both in the scenario of patients without heart disease (preventive effect), as in those with impaired systolic function.
With technological developments, is there a best strategy for cardiac pacing?
As of today, there is no unanimous definition of the best system, mode of operation or anatomic site for ACP[19]. Among the vulnerable individuals, the unfavorable evolution for the appearance of heart disease because of ACP could be linked to the interaction of at least 3 factors: (1) patient-substrate: normal heart versus pre-existing heart disease and systolic dysfunction (LVEF< 45 %)[13,15,18,27,31,48,49]; (2) ACP time and dose (%CVP): worse if >40% of time[32,35,46,50]; and (3) degree of dyssynchrony generated (ventricular lead site[4849,51,52], diQRS generated[53] and artificial LBBB[54]).
The interposition of RV pacing in individuals with pre-existing systolic dysfunction (variable proportion of those who receive a conventional PPM, but a significant group within those who implant ICDs)[21] accelerates the onset of heart disease by ACP and it is probably in this group that ACP of the RV should be minimized or even eliminated, when possible[52,55]. In search of a better clinical outcome, we grouped the ways of using ACP in two sets:
• Using algorithms to minimize RV pacing through the manipulation of the iAV (programming of the device);
• Using variants of cardiac stimulation systems: a) atrial single-chamber (AAI); b) alternative sites for the electrode implant; c) multisite pacing: biventricular and bifocal of the RV.
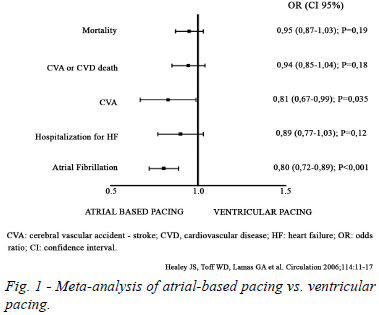
Most of the algorithms that minimize the ACP of the RV are basically looking for functionality in the AAI-R mode. Some of them are complemented with backup ventricular pacing (change to the DDD-R mode). However, these algorithms can at the same time produce AV decoupling and dyssynchrony by allowing for long, unrestricted and non-physiological iAVs. A better AV synchrony optimizes the myocardial pump by allowing atrial contraction at the appropriate pre-ejective ventricular moment. It also maximizes the diastolic ventricular filling (pre-loading). The AV synchrony is also beneficial to the mitral valve functioning and maintains the atrial pressures at significantly lower levels[17]. AV dyssynchrony, on the other hand, reduces cardiac output at rest from 20% to up to 50%[6,56]. A meta-analysis of more than 7,000 patients (Figure 1), which compared the ACP based on atria (AAI operation and, therefore, intrinsic QRS) with conventional stimulation of the RV, did not reveal significant differences in mortality or in the combined outcome of stroke and mortality or hospitalization for HF[57]. In another publication it was demonstrated that the iPR and the dose (%) of atrial stimulation were independent predictors of AV dissociation. When AV decoupling occurred, the cumulative CVP increased 10 times in comparison to patients without this electromechanical effect[58].
In a comprehensive manner, without distinguishing between patients with normal systolic function and those with impaired LVEF, the current Guidelines[2-5] recommend (level of evidence C) the use of algorithms that are able to maximally exploit the intrinsic AV conduction. However, we are not sure if with this form of stimulation we are not at the same time, in certain contexts and patient profiles (iPR> 300 ms), changing the sequence of mechanical coupling between atria and ventricles[52]. The maximum limit of iAV before AV dyssynchrony appears, however, has not been determined exactly, and neither are there data available to establish how much the minimization of the stimulus of the RV, at the expense of AV dyssynchrony, outweighs the risk of artificial ventricular activation.
When alternative ACP strategies are considered, through single-chamber atrial functioning, either by placing a single electrode or by programming the AAI mode, one must face the non-negligible risk of symptomatic associated AVB, which incidence is variable in the literature. The Brazilian Guidelines[2] cite an incidence of 8.4% in this context. If the patient with SND has some other kind intraventricular conduction or atrial fibrillation disorder, the incidence increases to more significant values[59-62]. It is worth pointing out that the first clinical manifestation of AV dysfunction is syncope in up to 50% of cases[21], which, paradoxically, is one of the most important symptoms to be relieved by implantation of a PPM.
Endocardial cardiac pacing has traditionally been performed with implantation of the electrode at the apex of the RV because of the electrical stability and, above all, the surgical ease, since it is the most accessible transvenous way[18]. Virtually all studies on artificial ventricular pacing have shown some kind of influence of the activation site on the cardiac hemodynamics. For this reason, alternatives for the stimulation of different topographies instead of the apex of the right ventricle have been assessed, especially the outflow tract of the RV, a septal and para-Hissian location, or even the endocardial pacing of the LV. Studies in patients with SND and preserved AV conduction, with randomization for the apical versus the outflow tract of the RV, and that contemplated these aspects, demonstrated the superiority of the latter location in terms of LVEF and remodeling when the follow-up exceeded 18 months[63,64]. Because of the methodological differences for the confirmation of the position of the electrode, however, and because we know that a great part of the hemodynamic results would depend on the obtained diQRS[65], until the moment these findings have not been incorporated into the Guidelines.
Pachón et al.[66-68] used bifocal pacing of the RV (conventional electrodes in septum and apex) in an attempt to promote the resynchronization of the LV. Despite the relatively small number of patients, the authors observed a reduction in diQRS and mitral regurgitation, in addition to significant improvement of systolic and diastolic function, which resulted in functional and quality of life improvements for the individuals. They proposed this alternative for cases with less myocardial impairment, as would be the case in heart conditions exclusively secondary to ACP, and/or when CRT is not possible by placing the LV electrode via coronary sinus. Despite the apparent good results, the most recent Guidelines[4,5] also don't consider this proposal.
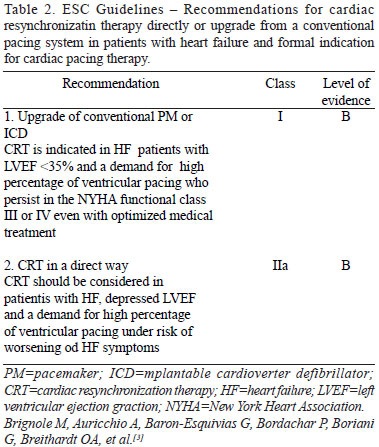
With the evidence and the clinical practice, an understanding is mounting of the role of primary prevention of HF by CRT. To prevent heart disease induced by ACP, there could be benefits to the upgrade to a multisite pacing system when is indicated to implant a device (PPM or ICD) and there is reduced when there is reduced LVEF (<35 or 40%), and the prediction of high cumulative CVP[3-5] (Table 2). While the European Guidelines[3] do not establish a definite value for CVP, the American Guidelines[5] mention that it must exceed 40% for a recommendation IIa. The American Guidelines also state that despite the absence of symptoms at the time of elective replacement of the conventional PPM generator, when there is reduced LVEF, the upgrade to CRT could be associated with a better outcome[65,69,70].
However, from the same perspective that the role of multisite pacing is more a preventive than a therapeutic strategy, the theory arises that in patients with preserved or less compromised systolic function, CRT could revert the dyssynchrony artificially produced by the action of ACP[69,71]. There is also evidence[14,72] that it would be plausible to prescribe CRT for these patients since the first implant. A prospective and randomized study[73] reported the superiority of CRT in comparison to apical RV pacing in patients with preserved LVEF and bradycardia. A post-hoc analysis of the MADIT-CRT[74] found up to 38% of patients with EF>30% (ranging from 30-45.3%), with these patients having significant clinical benefits with CRT and, surprisingly, showing an even more pronounced reduction of the risk of events when compared to those with more depressed LVEF (reduction of 44% of primary outcomes, including mortality from any cause).
Another study[75] that compared conventional pacing in complete AVB with CRT in 918 patients with a mean LVEF of 40%, showed that the combined outcome of death from any cause, emergency treatment for HF, and increase in end-systolic volume of the LV, was less frequent with biventricular pacing (53.3% vs. 64.3%). The result of this study suggests that CRT supports the ventricular function in patients who require ACP and with moderately reduced LVEF. These data were supported by the PACE study[71], which demonstrated that the potential deleterious effects of RV apical pacing could be avoided with CRT in patients with moderately reduced LVEF.
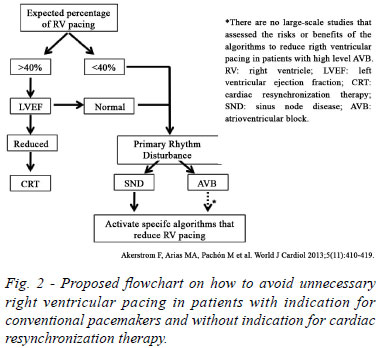
In conclusion, is there a best strategy for cardiac pacing? The analyzed literature reports that the vast majority of patients with a structurally normal heart and preserved systolic function tolerates, in varying degrees, any changes generated by ACP of the RV. This is possible due to the operation of mechanisms of the myocardial functional reserve. When there is a prior impairment of systolic function and, probably, these compensatory mechanisms have been exhausted, the set of alterations, such as the increase of diQRS, ventricular dyssynchrony and the artificially generated LBBB, gives rise to the ventricular pacemaker syndrome[6] and clinically manifest HF. Therefore, when the implantation of a device is indispensable, one must thoroughly assess the patient-substrate set, the systolic function and the AV conduction in order to estimate the cumulative CVP and to establish, based on this, the decision flow (Figure 2)[52]. In patients with AV dyssynchrony and a structurally normal heart (preserved LVEF), a programming that allows for non-physiological iAVs or that may cause AV uncoupling, may not be suitable. When the AV conduction is preserved, one should look to minimize RV pacing by the use of specific algorithms, also trying not to generate paradoxical AV dyssynchrony. If ACP of the RV is mandatory, and if there is no concomitant systolic dysfunction or structural heart disease, we can be confident that the probability of the patient progressing to HF by ACP is low[13,15,22,23,27,37,39]. In these patients, one can try to implant the electrode in a septal position or in the RV outflow tract in order to obtain a more favorable activation vector with greater probability of capturing the intrinsic conduction system and producing lower diQRS. In patients with LVEF <50% and anticipation of high CVP percentage (>40%), CRT should be stratetic choice.
CONCLUSION
ACP is a safe and effective therapy for the treatment of irreversible bradyarrhythmias, but it is not devoid of potential adverse effects. Patients who receive devices for conventional indications, and who have structurally normal hearts and preserved LVEF, often present less secondary heart diseases from ACP, even with high doses of cumulative artificial activation (%CVP) of the RV. In the absence of a substrate and predisposing factors, some doubt remains about which clinical, hemodynamic, structural or even genetic circumstances we should care for to avoid heart disease induced by ACP of the RV. Cardiac resynchronization therapy emerges, in the context of dyssyncropathy, extended diQRS, artificial LBBB pattern and %CVP by ACP, as the most promising option for treatment, while the role of other strategies remains controversial.
REFERÊNCIAS
1. Kalil C, Nery PB, Bartholomay E, Albuquerque LC. Tratamento com cardioversor-desfibrilador implantável e ressincronização cardíaca: isolados ou associados? Rev Bras Cir Cardiovasc. 2006;21(1):85-91. Visualizar artigo
2. Martinelli Filho M, Zimerman LI, Lorga AM, Vasconcelos JTM, Rassi A Jr. Guidelines for Electronic Cardiac Implantable Devices of the Brazilian Society of Cardiology. Arq Bras Cardiol. 2007;89(6):e210-e238.
3. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. The Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Eur Heart J. 2013;34(29):2281-329. [MedLine]
4. Gillis AM, Russo AM, Ellenbogen KA, Swerdlow CD, Olshansky B, Al-Khatib SM, et al.; Heart Rhythm Society; American College of Cardiology Foundation. HRS/ACCF expert consensus statement on pacemaker device and mode selection. Developed in partnership between the Heart Rhythm Society (HRS) and the American College of Cardiology Foundation (ACCF) and in collaboration with the Society of Thoracic Surgeons. Heart Rhythm. 2012;9:1344-65. [MedLine]
5. Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA 3rd, et al. 2012 ACCF/AHA/HRS Focused Update of the 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60(14):1297-313. [MedLine]
6. Pachón Mateos J, Pachón Mateos EI, Mattos Barreto RBl. Estimulação Cardiaca na Insuficiência Cardíaca e Ressincronização. In: Pachón Mateos JC, ed. Marca-passos, Desfibriladores e Ressincronizadores Cardíacos: Noções Fundamentais para o Clínico. 1ª Ed. São Paulo: Atheneu 2014.
7. Toff WD, Camm AJ, Skehan JD; United Kingdom Pacing and Cardiovascular Events Trial Investigators. Single-chamber versus dual-chamber pacing for high-grade atrioventricular block. N Engl J Med. 2005;353(2):145-55. [MedLine]
8. Teno LAC, Costa R, Martinelli Filho M, Castilho FCT, Ruiz I, Stella UB, et al. Efeitos da mudança de modo de estimulação ventricular para atrioventricular sobre a qualidade de vida em pacientes com cardiopatia chagásica e bloqueio atrioventricular na troca eletiva do gerador de pulsos. Rev Bras Cir Cardiovasc. 2005;20(1):23-32. Visualizar artigo
9. Vardas PE, Simantirakis EN, Kanoupakis EM. New developments in cardiac pacemakers. Circulation. 2012;127(23):2343-50.
10. Jutzy RV, Feenstra L, Pai R, Florio J, Bansal R, Aybar R, et al. Comparison of intrinsic versus paced ventricular function. Pacing Clin Electrophysiol. 1992;15(11 Pt 2):1919-22. [MedLine]
11. Vogler J, Breithardt G, Eckardt L. Bradyarrhythmias and conduction blocks. Rev Esp Cardiol. 2012;65(7):656-67.
12. Manolis AS. The deleterious consequences of right ventricular apical pacing. Pacing Clin Electrophysiol. 2006;29(3):298-315. [MedLine]
13. Sweeney MO, Prinzen FW. Ventricular pump function and pacing: physiological and Clinical Integration. Circ Arrhythmia Electrophysiol. 2008;1(2):127-39.
14. Fang F, Zhang Q, Chan JY, Razali O, Azlan H, Chan HC, et al. Early pacing-induced systolic dyssynchrony is a strong predictor of left ventricular adverse remodeling: analysis from the pacing to avoid cardiac enlargement (PACE) trial. Int J Cardiol. 2013;168(2):723-8. [MedLine]
15. Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dissyncrhony implications for therapy. J Am Coll Cardiol. 2009;54(9):764-6.
16. Tanabe A, Mohri T, Ohga M, Yoshiga O, Hidaka Y, Ikeda H, et al. The effects of pacing-induced left bundle branch block on left ventricular systolic and diastolic performances. Jpn Heart J. 1990;31(3):309-17. [MedLine]
17. Barold S, Ovsyshcher E. Pacemaker-induced mitral regurgitation. Editorial. Pacing Clin Electrophysiol. 2005;28(5):357-60. [MedLine]
18. Pachón Mateos JC, Pachón Mateos EI, Pachón Mateos JC. Right ventricular apical pacing: the unwanted model of cardiac stimulation? Expert Rev Cardiovasc Ther. 2009;7(7):789-99. [MedLine]
19. Sanaa I, Francheschi F, Prevot S, Bastard E, Deharo JC. Right Ventricular apex pacing: is it obsolete? Arch Cardiovasc Dis. 2009;102(2):135-41. [MedLine]
20. Trohman RG, Kim MH, Pinski SL. Cardiac pacing: the state of the art. Lancet. 2004;364(9446):1701-19. [MedLine]
21. Sweeney MO, Prinzen FW. A new paradigm for physiological ventricular pacing. J Am Coll Cardiol. 2006;47(2):282-8 [MedLine]
22. Garillo R, Alvarez MM. Marcapasos cardiacos. Estimulación desde el ventrículo derecho: Beneficios y perjuicios a la luz de la experiência actual. Rev Costarric Cardiol. 2011;13(1):19-22.
23. Nahlawi M, Waligora M, Spies SM, Bonow RO, Kadish AH, Goldberger JJ. Left ventricular function during and after right ventricular pacing. J Am Coll Cardiol. 2004;44(9):1883-8. [MedLine]
24. Moreira Neto FF, Engel A, Sgarbieri NR, Bombonato R, Brasil JCF. Implante de marcapasso ventricular esquerdo no tratamento da miocardiopatia dilatada e bloqueio de ramo esquerdo associado a discinesia de contração septal. Rev Bras Cir Cardiovasc. 1998.13(3):256-62. Visualizar artigo
25. Vassallo JA, Cassidy DM, Miller JM, Buxton AE, Marchlinski FE, Josephson ME. Left ventricular endocardial activation during right ventricular pacing: effect of underlying heart disease. J Am Coll Cardiol. 1986;7(6):1228-33. [MedLine]
26. O'Keefe JH, Abuissa H, Jones PG, Thompson RC, Bateman TM, McGhie AI, et al. Effect of chronic right ventricular apical pacing on left ventricular function. Am J Cardiol. 2005;95(6):771-3. [MedLine]
27. Hori Y, Tada H, Nakamura K, Naito S, Nakata Y, Goto K, et al. Presence of structural heart disease and left ventricular dysfunction predict hospitalizations for new-onset heart failure after right ventricular apical pacing. Europace. 2011;13(2):230-6. [MedLine]
28. Xiao HB, Brecker SJ, Gibson DG. Differing effects of right ventricular pacing and left bundle branch block on left ventricular function. Br Heart J. 1993;69(2):166-73 [MedLine]
29. Zhang Q, Fang F, Yip GW, Chan JY, Shang Q, Fung JW, et al. Difference in prevalence and pattern of mechanical dyssincrhony in left bundle branch block occurring in right ventricular apical pacing versus systolic heart failure. Am Heart J. 2008;156(5):989-95. [MedLine]
30. Funck RC, Blanc JJ, Mueller HH, Schade-Brittinger C, Bailleul C, Maisch B; BioPace Study Group. BioPace Study Group. Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the 'Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (BioPace)' study. Europace. 2006;8(8):629-35. [MedLine]
31. Sweeney MO, Hellkamp AS. Heart failure during cardiac pacing. Circulation. 2006;113(17):2082-8. [MedLine]
32. Wilkoff BL, Cook JR, Epstein AE, Greene HL, Hallstrom AP, Hsia H, et al.; Dual Chamber and VVI Implantable Defibrillator Trial Investigators. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial. JAMA. 2002;288(24):3115-23. [MedLine]
33. Sgarbossa EB, Pinski SL, Maloney JD, Simmons TW, Wilkoff BL, Castle LW, et al. Chronic atrial fibrillation and stroke in paced patients with sick sinus syndrome. Relevance of clinical characteristics and pacing modalities. Circulation. 1993;88(3):1045-53. [MedLine]
34. Sgarbossa EB, Pinski SL, Maloney JD. The role of pacing modality in determining long-term survival in the sick sinus syndrome. Ann Intern Med. 1993;119(5):359-65. [MedLine]
35. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, et al.; MOde Selection Trial Investigators. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107(23):2932-7. [MedLine]
36. Sgarbossa EB, Pinski SL, Trohman RG, Castle LW, Maloney JD. Single-chamber ventricular pacing is not associated with worsening heart failure in sick-sinus syndrome. Am J Cardiol. 1994; 73(9):693-97.
37. Riahi S, Nielsen JC, Hjortshøj S, Thomsen PE, Højberg S, Møller M, et al.; DANPACE Investigators. Heart failure in patients with sick sinus syndrome treated with single lead atrial or dual-chamber pacing: no association with pacing mode or right ventricular pacing site. Europace. 2012;14(10):1475-82. [MedLine]
38. Lamas GA, Lee KL, Sweeney MO, Silverman R, Leon A, Yee R, et al.; Mode Selection Trial in Sinus-Node Dysfunction. Ventricular pacing or dual-chamber pacing for sinus node dysfunction. N Engl J Med. 2002;346(24):1854-62. [MedLine]
39. Calvo Cuervo D, Rubín Lopez JM, Pachón Rebollo N. Minimizar la estimulación em el ventrículo derecho: por que, cuanto y en que paciente. Una revisión clínica. Cuad Estimul Card. 2012;5(14):41-6.
40. Shukla HH, Hellkamp AS, James EA, Flaker GC, Lee KL, Sweeney MO, et al.; Mode Selection Trial (MOST) Investigators. Heart failure hospitalization is more common in pacemaker patients with a prolonged paced QRS duration. Heart Rhythm. 2005;2(3):245-51. [MedLine]
41. Yu CM, Chan JY, Zhang Q, Omar R, Yip GW, Hussin A, et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med. 2009;361(22):2123-34. [MedLine]
42. Thambo JB, Bordachar P, Garrigue S, Lafitte S, Sanders P, Reuter S, et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation 2004;110(25):3766-72. [MedLine]
43. Dreger H, Maethner K, Bondke H, Baumann G, Melzer C. Pacing-induced cardiomyopathy in patients with right ventricular stimulation for > 15 years. Europace. 2012;14(2):238-42. [MedLine]
44. Sagar S, Shen WK, Asirvatham SJ, Cha YM, Espinosa RE, Friedman PA, et al. Effect of long-term right ventricular pacing in young adults with structurally normal heart. Circulation. 2010;121(15):1698-705. [MedLine]
45. Varma N. Left ventricular conduction delays induced by right ventricular apical pacing: effect of left ventricular dysfunction and bundle branch block. J Cardiovasc Electrophysiol. 2008;19(2):114-22. [MedLine]
46. Steinberg JS, Fischer A, Wang P, Schuger C, Daubert J, McNitt S, et al.; MADIT II Investigators. The clinical implications of cumulative right ventricular pacing in the multicenter automatic defibrillator trial II. J Cardiovasc Electrophysiol. 2005;16(4):359-65. [MedLine]
47. Olshansky B, Day JD, Lerew DR, Brown S, Stolen KQ; INTRINSIC RV Study Investigators. Eliminating right ventricular pacing may not be best for patients requiring implantable cardioverter-defibrillators. Heart Rhythm. 2007;4(7):886-91. [MedLine]
48. Schmidt M, Brömsen J, Herholz C, Adler K, Neff F, Kopf C, et al. Evidence of left ventricular dyssyncrhony resulting from right ventricular pacing in patients with severely depressed left ventricular ejection fraction. Europace. 2007;9(1):34-40. [MedLine]
49. Thackray SD., Witte KK, Nikitin NP, Clark AL, Kaye GC, Cleland JG. The prevalence of heart failure and asymptomatic left ventricular systolic dysfunction in a typical regional pacemaker population. Eur Heart J. 2003;24(12):1143-52. [MedLine]
50. Sweeney MO, Bank AJ, Nsah E, Koullick M, Zeng QC, Hettrick D, et al.; Search AV Extension and Managed Ventricular Pacing for Promoting Atrioventricular Conduction (SAVE PACe) Trial. Minimizing ventricular pacing to reduce atrial fibrillation in sinus-node disease. N Engl J Med. 2007;357(10):1000-8. [MedLine]
51. Barsheshet A, Moss AJ, McNitt S, Jons C, Glikson M, Klein HU, et al.; MADIT-II Executive Committee. Long-term implications of cumulative right ventricular pacing among patients with an implantable cardioverter-defibrillator. Heart Rhythm. 2011;8(2):212-8. [MedLine]
52. Akeström F, Arias MA, Pachón M, Jiménez-López J, Puchol A, Juliá-Calvo J. The importance of avoiding unnecessary right ventricular pacing in clinical practice. World J Cardiol. 2013;5(11):410-9. [MedLine]
53. Wang NC, Maggioni AP, Konstam MA, Zannad F, Krasa HB, Burnett JC Jr, et al.; Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) Investigators. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299(22):2656-66. [MedLine]
54. Olshanksky B. Wide QRS, narrow QRS. What's the difference? J Am Coll Cardiol. 2005;46(2):317-9. [MedLine]
55. Ahmed M, Gorcsan J 3rd, Marek J, Ryo K, Haugaa K, R Ludwig D, et al. Right ventricular apical pacing-induced left ventricular dyssynchrony is associated with a subsequent decline in ejection fraction. Heart Rhythm. 2014;11(4):602-8. [MedLine]
56. Syed FF, Hayes DL, Asirvatham SJ, Friedman PA. Hemodynamics of cardiac pacing: optimization and programming to enhance cardiac function. In: Hayes DL, Asirvatham SJ, Friedman PA, eds. Cardiac pacing, defibrillation and resynchronization: a clinical approach. Hoboken: Wiley-Blackwell; 2008.
57. Healey JS, Toff WD, Lamas GA, Andersen HR, Thorpe KE, Ellenbogen KA, et al. Cardiovascular outcomes with atrial-based pacing compared with ventricular pacing: meta-analysis of randomized trials, using individual patient data. Circulation. 2006;114(1):11-7. [MedLine]
58. Sweeney MO, Ellenbogen KA, Tang AS, Johnson J, Belk P, Sheldon T. Severe atrioventricular decoupling, uncoupling, and ventriculoatrial coupling during enhanced atrial pacing: incidence, mechanisms, and implications for minimizing right ventricular pacing in ICD patients. J Cardiovasc Electrophysiol. 2008;19(11):1175-80. [MedLine]
59. Sutton R, Kenny R. The natural history of sick sinus syndrome. Pacing Clin Electrophysiol. 1986; 9(6 Pt 2):1110-4. [MedLine]
60. Brandt J, Anderson H, Fåhraeus T, Schuller H. Natural history of sinus-node disease treated with atrial pacing in 213 patients: implications for selection of stimulation mode. J Am Coll Cardiol. 1992;20(3): 633-9. [MedLine]
61. Haywood GA, Ward J, Ward DE, Camm AJ. Atrioventricular Wenckebach point and progression to atrioventricular block in sinoatrial disease. Pacing Clin Electrophysiol. 1990;13(12 Pt 2):2054-8. [MedLine]
62. Andersen HR, Nielsen JC, Thomsen PE, Thuesen L, Vesterlund T, Pedersen AK, et al. Atrioventricular conduction during long-term follow-up of patients with sick sinus syndrome. Circulation. 1998;98(13):1315-21. [MedLine]
63. Vancura V, Wichterle D, Melenovsky V, Kautzner J. Assessment of optimal right ventricular pacing site using invasive measurement of left ventricular systolic and diastolic function. Europace. 2013;15(10):1482-90. [MedLine]
64. Mabo P, Gras D, Degand B. Jean Dupuis J, Pellissier A, Solnon A, et. Septal Right Ventricular lead position in and optimized DDD pacing in sinus node disease: comparison to preserved intrinsic conduction - the OPTIMIST Trial. Poster Session Heart Rhythm. 2014; May 9th [Acessed Jun 6, 2014]. Available from: http://ondemand.hrsonline.org/common/presentation-detail.aspx/15/35/1293/8587
65. Spanish Society of Cardiology Working Group for the 2013 ESC Guidelines on Cardiac Pacing and Cardial Resynchronization Therapy, Fernández Lozano I, Mateas FR, Osca J, Sancho Tello MJ, García Bolao I, et al. Comments on the 2013 ESC Guidelines on Cardiac Pacing and Cardiac Resynchronization Therapy. Rev Esp Cardiol. 2014;67(1):6-14.
66. Pachón Mateos JC, Albornoz RN, Pachón Mateos EI, Gimenez VM, Pachón MZ, Santos Filho ER, et al. Right ventricular bifocal stimulation in the treatment of dilated cardiomyopathy with heart failure. Arq Bras Cardiol. 1999;73(6):485-98. [MedLine]
67. Pachón JC, Pachón EI, Albornoz RN, Pachón JC, Kormann DS, Gimenes VM, et al. Ventricular endocardial right bifocal stimulation in the treatment of severe dilated cardiomyopathy heart failure with wide QRS. Pacing Clin Electrophysiol 2001;24(9 Pt 1):1369-76. [MedLine]
68. Pachón Mateos JC, Albornoz Vargas R, Pachón Mateos EI, Gimenez VM, Pachón MZC, Pachón Mateos JC, et al. Estimulação ventricular bifocal no tratamento da insuficiência cardíaca com miocardiopatia dilatada. Rev Bras Cir Cardiovasc. 2000;15(1):44-54. Visualizar artigo
69. Acena M, Regoli F, Auricchio A. Cardiac resynchronization therapy. Indications and contraindications. Rev Esp Cardiol. 2012;65(9):843-9.
70. Gierula J, Cubbon RM, Jamil HA, Byrom R, Baxter PD, Pavitt S, et al. Cardiac resynchronization therapy in pacemaker-dependent patients with left ventricular dysfunction. Europace. 2013;15(11):1609-14. [MedLine]
71. Nazeri A, Massumi A, Rasekh A, Saeed M, Frank C, Razavi M. Cardiac resynchronization therapy in patients with right ventricular pacing-induced cardiomyopathy. Pacing Clin Electrophysiol. 2010;33(1):37-40. [MedLine]
72. Chen S, Yin Y, Lan X, Liu Z, Ling Z, Su L, et al.; PREDICT-Heart Failure study international group. Paced QRS duration as a predictor for clinical heart failure events during right ventricular apical pacing in patients with idiopathic complete atrioventricular block: results from an observational cohort study (PREDICT-HF). Eur J Heart Fail. 2013;15(3):352-9. [MedLine]
73. Yu CM, Chan JY, Zhang Q, Omar R, Yip GW, Hussin A, et al. Biventricular pacing in patients with bradycardia and normal ejection fraction. N Engl J Med 2009;361(22):2123-34. [MedLine]
74. Kutyifa V, Hall WJ, Solomon S, Knappe D, Pouleur AC; Scott McNitt, et al. Predictors of dyssynchrony and its role in subsequent outcome in CRT-D patients: results from the long-term follow-up of MADIT-CRT. J Am Coll Cardiol. 2013;61(10_S) doi: 10.1016/S0735-1097(13)61087-6. [MedLine]
75. Curtis AB, Worley SJ, Adamson PB, Chung ES, Niazi I, Sherfesee L, et al.; Biventricular versus Right Ventricular Pacing in Heart Failure Patients with Atrioventricular Block (BLOCK HF) Trial Investigators. biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368(17):1585-93. [MedLine]
No financial support.
Authors' roles & responsibilities
ADLF: Analysis and/or interpretation of data, Final approval of the manuscript
Conception and design of the study, Implementation of the operations and/or experiments, Drafting of the manuscript or critical review of its contents
APB: Analysis and/or interpretation of data, Final approval of the manuscript
Conception and design of the study, Implementation of the operations and/or experiments, Drafting of the manuscript or critical review of its contents
LCA: Final approval of the manuscript, Drafting of the manuscript or critical review of its contents
CPS: Analysis and/or interpretation of data, Implementation of the operations and/or experiments
PRR: Analysis and/or interpretation of data, Implementation of the operations and/or experiments
RMP: Analysis and/or interpretation of data, Drafting of the manuscript or critical review of its contents
MW: Final approval of the manuscript, Drafting of the manuscript or critical review of its contents
MAG: Implementation of the operations and/or experiments, Drafting of the manuscript or critical review of its contents
Article receive on segunda-feira, 7 de abril de 2014
 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license