Xue-Shan HuangI; Zeng-Rong LuoI; Qiang ChenI; Ling-Shan YuI; Hua CaoI; Liang-Wan ChenI; Gui-Can ZhangI
DOI: 10.21470/1678-9741-2018-0351
ABSTRACT
Objectives: To compare the advantages and disadvantages of perventricular and percutaneous procedures for treating isolated ventricular septal defect (VSD).AVB = Atrioventricular block
AR = Aortic regurgitation
ICU = Intensive care unit
LA = Left atrium
LV = Left ventricle
PFO = Patent foramen ovale
RA = Right atrium
RV = Right ventricle
TEE = Transesophageal echocardiography ok
TTE = Transthoracic echocardiography
VSD = Ventricular septal defect
INTRODUCTION
Ventricular septal defect (VSD) is one of the most common congenital heart diseases in both children and adults. Different types of VSDs exist based on different anatomical positions, and perimembranous ventricular septal defects account for 70% to 80% of all VSDs[1]. Although surgical repair is the standard treatment for isolated VSD and can achieve a satisfactory curative effect, it is associated with scarring, postoperative pain, a long hospital stay, and organ dysfunction, which may cause physical and psychological trauma to patients. Catheter-based therapy for VSD closure has become another standard treatment for this disorder[2]. Currently, because of improved technology, percutaneous device closure approaches are becoming increasingly popular for VSD treatment, and encouraging results have been observed[3-5]. In recent years, perventricular device closure for isolated VSD has being developed, especially in China[6-8]. Similar to the success of percutaneous closure of isolated VSD, this approach has also achieved good clinical outcomes. This study is based on our own single-institutional experience and aims to compare the advantages and disadvantages of these two procedures in treating isolated VSD.
METHODS
The authors declare that they have no competing interests. The study was approved by the ethics committee of our university and adhered to the Declaration of Helsinki. Additionally, all patients were fully informed about the advantages and disadvantages of both procedures, and written informed consent was obtained from the patients and the patients’ families.
Patients
From January 2015 to December 2016, we enrolled 572 patients with isolated VSD who underwent perventricular or percutaneous device closure in our center, and they were divided into two groups according to the treatment method that they selected. The patient flow chart is shown in Figure 1, and all the patients’ clinical data are shown in Table 1. No statistically significant differences in gender, age, and body weight distribution were noted between the two groups.
| Group A | Group B | P -value | |
|---|---|---|---|
| Number of patients | 412 | 135 | |
| Male/Female | 201/211 | 64/71 | 0.525 |
| Age (years) | 6.2±6.3 | 6.5±5.2 | 0.432 |
| Body weight (kg) | 33.1±21.5 | 31.5±23.4 | 0.608 |
| VSD size (mm) | 5.9 ± 2.3 | 6.1±2.1 | 0.761 |
| Operative time (minutes) | 32.5±12.6 | 70.2±25.5 | 0.034 |
| Immediately closure rate | 98.5% | 97.7% | 0.544 |
| Fluoroscopy time (minutes) | 0 | 32.1±21.5 | 0.010 |
| ICU stay (hours) | 12.5±8.3 | 0 | 0.022 |
| Hospital stay (days) | 3.8±2.1 | 3.1±1.9 | 0.894 |
| Follow-up (years) | 1.3±0.7 | 1.2±0.9 | 0.962 |
The inclusion criteria were: (1) isolated VSD; (2) a significant hemodynamic left to right shunt, and/or significant chamber enlargement, and/or mild pulmonary arterial hypertension; (3) no aortic regurgitation and a subaortic rim of the VSD greater than 2 mm; (4) concomitant symptoms such as chest pain, shortness of breath, activity limitation, palpitation, or repeated respiratory tract infection; (5) age greater than one year in group A and greater than three years in group B. The exclusion criteria were: (1) severe aortic valve prolapse and moderate-severe aortic regurgitation; (2) other congenital heart diseases requiring surgical repair; (3) infectious diseases that had not been cured, uncontrolled congestive heart failure, a neoplasm in the heart cavity, VSD location that would cause treatment failure, hemorrhagic disease, and evident dysfunction of liver and kidneys; (4) the presence of a thrombus in the operative route in group B.
All patients underwent routine clinical examinations, including a standard lead electrocardiogram, chest radiography, routine blood and biochemistry tests, and a coagulation function test. In both groups, 55 patients suffered from palpitations, shortness of breath, and chest tightness, but all of them showed good cardiac function and exercise tolerance. The diagnosis of VSD was determined by transthoracic echocardiography (TTE), and the diameter of VSD was measured by TTE on multiple views. In group A, 65 patients suffered from mild pulmonary arterial hypertension, 40 of whom had a patent foramen ovale (PFO), while 34 patients had concurrent mild tricuspid regurgitation. These data in terms of proportions were similar to those in group B, where 19 patients had mild pulmonary arterial hypertension, 10 patients had a PFO, and 11 patients had mild tricuspid regurgitation. No moderate-severe aortic regurgitation was noted in either group. Because most of the patients had isolated VSD, cardiac catheterization was not part of the routine examination.
Device
The patients in group B underwent percutaneous VSD device closure with the Amplatzer VSD occluder (AGA Medical, Corporation, Plymouth, Minn), while those in group A were treated with minimally invasive perventricular device closure, with a device produced domestically, which was manufactured by Lifetech Scientific (Shenzhen) Co, Ltd. (Shanghai Shape Memory Alloy Co, Ltd.) and Shan Dong Visee Medical Apparatus Co., Ltd. in China (Figure 2). The delivery system in group B consists of an approximately sized VSD occluder (produced from an alloy of nickel and titanium alloy), a trocar, a guidewire, delivery sheaths (outer and inner), a loading sheath, and a device cable. Both asymmetric and symmetric occluders were used in both groups. With the asymmetric occluder, the aortic end of the disk is 0-1 mm wider than the waist of the left ventricular side, with a platinum marker to guide device orientation, and the other side is 5-6 mm wider than the waist. With the symmetric device, the left and right ventricular disks are both 2 mm larger than the waist. An appropriate device size (1-2 mm larger than the defect) was selected based on the VSD size assessed by angiography or echocardiography.
Protocol
The patients in group A underwent minimally invasive perventricular device closure. General anesthesia was administered to the patients, and they were then placed in the supine position and draped for exposure of the entire chest. Transesophageal echocardiography (TEE)/TTE was performed to guide the procedure. A lower partial median sternotomy was performed, and the pericardium was opened to expose the right ventricle. The puncture site was selected by gently pressing the right ventricular free wall with a finger under continuous TEE monitoring, thus ensuring that the puncture site was perpendicular to the defect and free of internal cardiac tissue. Two parallel 5/0 or 4/0 Prolene sutures of U-stitches were stitched around the puncture site. Heparin was administered to the patients (1 mg/kg, intravenously), and the activated clotting time was monitored and maintained above 250 seconds at the beginning of the procedure. A modified short angiocatheter was passed into the right ventricle, and then the needle was removed. A floppy guidewire was advanced through the angiocatheter into the right ventricle and then passed through the defect into the left ventricle. Then, the trocar was removed. An appropriately sized delivery sheath was advanced over the guidewire into the left ventricle. The wire and the inner sheath of the delivery sheath were then removed, and the sheath was allowed to back bleed to ensure the absence of air entrapment. The VSD occluder was screwed onto the delivery cable and then pushed into the loading sheath. The loading sheath was introduced into the delivery sheath, and the occluder was advanced slowly through the sheath. The left disk was deployed under continuous TEE guidance. Next, the sheath was pulled back slowly until the left disk approached the ventricular septum. Then, the waist and right ventricle disks were deployed consecutively with sustained traction on the delivery cable in their corresponding locations (Figure 3). If no significant residual shunt (a significant residual shunt indicates that a greater than 75% pressure gradient still exists after occluder release), aortic regurgitation, or tricuspid regurgitation was observed, then the occluder was released. During implantation of an asymmetric device, the platinum marker of the distal disk was far from the aortic valve and pointed downward. The delivery sheath was withdrawn with the suture securely tied. The chest was closed routinely with drainage tube placement. An oral anticoagulant drug (dipyridamole or aspirin) was supplied to prevent thrombus formation in the patients who underwent the operation[7].
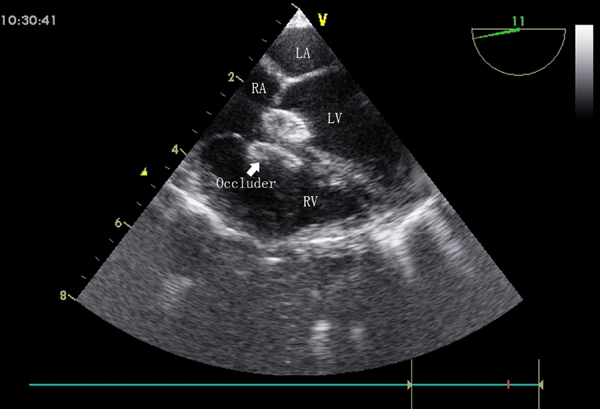
The patients in group B underwent percutaneous device closure in a catheterization operating room under general anesthesia and radiography guidance. Heparin was administered intravenously immediately before the procedure. A catheter was inserted into the left and right heart to assess ventricular pressure and pulmonary vascular resistance. Ascending aortic and left ventricular angiography was performed to evaluate the degree of shunting, the location of the VSD, and the function of the aortic valve. Then, the defect was crossed from the left to the right ventricle by a right coronary catheter with the help of a guidewire. Next, the guidewire was replaced by a soft exchange wire, which was advanced to the pulmonary artery branch. Then, the exchange wire was snared in the pulmonary artery using a gooseneck snare, and this wire was exteriorized through the femoral vein to establish a stable arteriovenous loop. A delivery sheath was advanced from the femoral vein into the left ventricular cavity and positioned in the ascending aorta. An appropriately sized occluder was selected, screwed onto the delivery cable, and advanced gently through the sheath until the occluder reached the tip of the sheath. The sheath was then slowly pulled back from the apex and the occluder was released under radiographic guidance (Figure 4). Left ventricular and ascending aortic angiography and TTE were repeated to verify the correct location of the occluder.
Statistical Analysis
Continuous data are presented as mean ± standard deviation and range. Clinical parameters of both groups were compared with independent-samples t-test. The Wilcoxon rank-sum test was used for data without a normal distribution. Nominal variables were compared between the two groups using Fisher's exact test. A P-value < 0.05 was defined as statistically significant.
RESULTS
Overall, 412 patients in group A underwent successful closure, while the remaining 15 cases were converted to conventional surgical repair because of new moderate-severe aortic valve regurgitation (six patients), failure to establish a transfer orbit (one patient), occluder dislodgement (two patients), significant residual shunt (five patients), and immediate Mobitz type II atrioventricular block (AVB) (one patient). In the successful cases, a symmetric occluder was used in 348 patients (84.5%), and an asymmetric occluder was used in 64 patients (15.5%). The diameter of the VSD ranged from 5 to 10 mm (5.9±2.3 mm), and the size of the implanted occluder ranged from 6 to 12 mm (6.6±2.8 mm). In group A, the successful VSD closure rate was 98.5% immediately after the operation and 99.5% in the 3-month follow-up. In comparison, 135 cases of successful closure were noted in group B, and 10 patients were converted to surgical repair due to new moderate-severe aortic valve regurgitation (five patients), occluder dislodgement (two patients), significant residual shunt (two patients), and immediate AVB (one patient). The successful VSD closure rates were not significantly different from those in group A (97.7% and 100%, respectively) (Tables 1 and 2).
| Reason | Group A | Group B |
|---|---|---|
| New moderate-severe AR | 6 | 5 |
| Failure to establish transfer orbit | 1 | |
| Occluder dislodgement | 2 | 2 |
| Significant residual shunt | 5 | |
| Immediate Mobitz type II AVB | 1 | 2 |
| Immediate AVB | 1 |
In group A, major complications occurred in some cases after the procedure (Table 3). During the procedure, Mobitz type II AVB occurred in one case, which was then converted to surgical repair. No complete AVB occurred in the perioperative period. A total of 12 patients developed new arrhythmias after the procedure, including temporary sinus bradycardia or tachycardia and transient bundle branch block, which either spontaneously resolved or were easily and successfully treated pharmacologically. Six patients were converted to surgical repair for new moderate-severe aortic valve regurgitation during the procedure. Additionally, five patients suffered trace to mild aortic valve regurgitation after the procedure, but showed no significant symptoms or progressive regurgitation during the follow-up. No relevant digestive or respiratory tract complications caused by the TEE probe occurred in this study.
| Complication | Group A | Group B |
|---|---|---|
| Early complete AVB | 0 | 1(0.7%) |
| Late-onset complete AVB | 1(0.2%) | 1(0.7%) |
| Newly mild AR | 5(1.2%) | 6(4.1%) |
| Newly moderate-severe AR | 6(1.4%) | 5(3.4%) |
| Transient arrhythmias | 12(2.8%) | 35(24.1%) |
| Pericardial effusion | 3(0.7%) | 0 |
| Pulmonary infection | 3(0.7%) | 0 |
| Hematoma at access site | 0 | 2(1.4%) |
| Trauma to femoral artery | 0 | 0 |
| Fat liquefaction of incision | 3(0.7%) | 0 |
In our comparative study, the patients in group A had longer intensive care unit (ICU) stay than those in group B (P<0.05), but the patients in group B experienced significantly longer operative times than those in group A (P<0.05). The hospital stay was almost the same.
During the follow-up period, both groups had one case of late-onset complete AVB. A permanent pacemaker was inserted in these two cases. All patients underwent TTE examinations, first at three months after the operation, and subsequent examinations were performed as required by clinical practice guidelines. Two patients had tiny residual shunts; one shunt in a group A patient was located at the edge of the occluder, but no further intervention was needed. No arterial damage or leg-shortening was observed in group B. Neither group had any other serious complications or mortality, such as cerebral embolism, cardiac perforation, cardiac valve distortion, endocarditis, or malignant arrhythmia. No additional medical treatment for PFO was provided in either group during the current follow-up period.
DISCUSSION
VSD is one of the most common types of congenital heart malformation and is usually accompanied by cardiac capacity overload, pulmonary arterial hypertension, congestive heart failure, infectious endocarditis, and even Eisenmenger syndrome, some of which can be life-threatening. Surgical closure is the gold standard treatment for all kinds of VSDs, which has yielded successful outcomes in recent years. This procedure enables direct visualization of the defect and a wide operating space for surgeons. However, surgical repair has the disadvantages of postoperative discomfort, long and unsightly surgical incision, wound pain, long hospital stay, and big psychological and physical impact on patients. In addition, cardiopulmonary bypass may lead to myocardial reperfusion injury and organ dysfunction[9-12]. With the popularization of minimally invasive techniques, percutaneous device closure of isolated VSDs has become another choice for patients. The safety and feasibility of this procedure have been reported by many medical centers, and the complications of this procedure seem to be limited according to various reports. However, percutaneous device closure requires X-ray exposure to both patients and doctors[13-15]. Recently, perventricular device closure of isolated VSDs has become increasingly popular, especially in China. This approach has the advantages of no X-ray exposure, simple execution, being easy to learn and master, short operative time, and minimal cosmetic incision[16,17]. From previous studies, we know that both percutaneous and perventricular device closure procedures are safe and effective for isolated VSDs and have respective advantages and disadvantages. Studies comparing percutaneous or perventricular device closure and surgical repair have been extensively reported. To our knowledge, very few related reports comparing these device treatments for isolated VSDs are available.
Articles with large-sample data analyses in relation to both percutaneous and perventricular device closures of VSDs are thoroughly established. Yang et al. shared their research results for transcatheter closure of isolated VSDs in 142 children using symmetric and asymmetric occluders. The success rates were 93.8% and 94.9% with asymmetric and symmetric occluders, respectively[18]. Yang et al. evaluated the safety and efficacy of transcatheter closure for isolated VSDs and the long-term results. Placement of the device was successful in 832 patients (98.1%). Nine major adverse events were reported (8.7%), including two cases of complete AVB requiring pacemaker implantation[3]. Xing et al.[6] summarized their clinical experiences and midterm follow-up results for perventricular closure of isolated VSDs. A total of 408 patients with isolated VSDs underwent this procedure and the successful closure rate was 96.3%. New trace or mild tricuspid regurgitation was found in 13 patients (3.3%), and seven patients (2.8%) had an incomplete right bundle branch block[6]. Yang Y et al.[19] reported that 889 children diagnosed with isolated VSDs underwent surgical occlusion using TEE for guidance. Their results showed that 94.37% of the children underwent successful closure. Symmetric devices were used in 741 cases and asymmetric devices were used in the remaining 148 cases[19]. From these reports, both methods can obtain good results, but which one should we choose?
Our results also confirmed that the two approaches yielded similar success rates and safety. Group B had fewer complications, no incisions, and did not require an ICU stay compared with group A. For many patients with isolated VSDs, percutaneous device closure can be their first treatment choice. However, for the patients in group A, the procedure was usually guided by TTE or TEE without X-ray exposure, which is a more attractive choice for patients who are unwilling or unable to be exposed to radiation. Another reason for selecting the perventricular procedure is that it provides a perpendicular angle from the right ventricle to the isolated ventricular septum, which may facilitate guidance of the wire through the VSD. The relatively short delivery sheath used in group A facilitated position adjustments and deployment of the occluder into the defect. For these reasons, this technique can simplify the procedure and is easy to implement, and the relatively short learning curve is suitable for beginners. The average procedure time was no longer than 1 hour, which is more acceptable to most operators and patients. Due to the lack of hybrid operating rooms in China, the percutaneous procedure was performed in a catheterization laboratory, while the perventricular procedure was conducted in an operating room. In group A, once the procedure failed, conversion to routine surgical repair was executed without additional delay.
We must emphasize that TEE and TTE play important roles in the perventricular closure method. Preoperative TEE or TTE can certainly show the type, size, and surrounding structures of the VSD, especially the distance from the defect to the aortic valves, reflecting the superiority of TEE and TTE to cardiac angiography. In our early experience, we used TEE as the guiding tool for our procedure[7]. With accumulated experience, we have found that TTE also provided distinct images for guidance in surgeries involving young children and infants[20]. Measuring the size of the VSD and evaluating the function of the aortic valve are key points in the procedure. The former step helps to determine an appropriate occluder size, which should be the same size as or 1-2 mm larger than the VSD. The latter step helps to determine whether to select an asymmetric occluder or terminate the procedure. The location and size of the occluder may affect the function of the aortic valves, which can lead to valvular anatomy damage and cardiac function failure in the future. In our opinion, the new occurrence of moderate-severe aortic regurgitation is an indicator to recommend surgery. Aortic valve regurgitation can disappear when the occluder is recycled, and no further aortic valve plasty is needed in such patients. For new mild aortic regurgitation, although we have a long follow-up experience and good clinical results, we still believe that the choice of whether to use the device closure should depend on the operator's clinical experience.
Many reports have focused on complete AVB occurring during and after device closure of VSDs, which is a catastrophic complication for patients. The AVB incidence was not the same as that in the reports using transcatheter device closure, which ranged from 1%-5%, but the exact reason remains speculative[21-23]. In this comparative study, the AVB incidence was less than 1% in both groups, indicating no statistically significant difference. Mechanical injury of the conduction system by the delivery system or device may be a reasonable cause of acute, early, complete AVB. Considering the convenience of the manipulation process, the possibility of the perventricular procedure causing such an injury is smaller than that for the transcatheter method. Because of our accumulated experience, early AVB in the perioperative period rarely occurred during this study. We still strongly recommend that the procedure should be converted to surgical repair once an early complete AVB occurs. For late-onset complete AVB, chronic inflammation or fibrosis may be a reasonable explanation. The chances of these two types of AVB occur are the same with both procedures. No effective preventive measures for late-onset complete AVB appear to be available; implantation of a permanent pacemaker seems to be the only option. Fortunately, the incidence does not seem to be high.
Limitations
Because the study was retrospective and the distribution of the enrolled patients was not randomized, a bias may be associated with the data collection. In addition, this study only focused on isolated VSDs, and other types of VSDs may result in different outcomes with these two treatments. In addition, the study was conducted in only one cardiac center and the sample size was limited. Other centers may obtain different results. Of course, a longer follow-up than ours is necessary to evaluate the long-term effect.
CONCLUSION
Based on this study, we can conclude that both perventricular and percutaneous device closure procedures are safe and effective in treating patients with isolated VSDs, but the percutaneous procedure has obvious advantages of less trauma compared to the perventricular approach. However, the perventricular procedure is simpler to execute and requires a shorter operative time than the percutaneous approach and avoids X-ray exposure. In a word, both procedures have their own advantages and disadvantages, and we should choose the appropriate treatment according to the patient's actual situation.
REFERENCES
1. Diab KA, Cao QL, Hijazi ZM. Device closure of congenital ventricularseptal defects. Congenit Heart Dis. 2007;2(2):92-103.doi:10.1111/j.1747-0803.2007.00080.x. [MedLine]
2. Minette MS, Sahn DJ. Ventricular septal defects. Circulation.2006;114(20):2190-7. Erratum in: Circulation. 2007;115(7):e205.doi:10.1161/CIRCULATIONAHA.106.618124.
3. Yang J, Yang L, Wan Y, Zuo J, Zhang J, Chen W, et al. Transcatheterdevice closure of perimembranous ventricular septal defects: mid-term outcomes.Eur Heart J. 2010;31(18):2238-45. doi:10.1093/eurheartj/ehq240.
4. Koneti NR, Sreeram N, Penumatsa RR, Arramraj SK, Karunakar V,Trieschmann U. Transcatheter retrograde closure of perimembranous ventricularseptal defects in children with the amplatzer duct occluder II device. J Am CollCardiol. 2012;60(23):2421-2. doi:10.1016/j.jacc.2012.08.1004. Erratum in: J AmColl Cardiol. 2013;61(5):598. doi:10.1016/j.jacc.2012.12.006.
5. Odemis E, Saygi M, Guzeltas A, Tanidir IC, Ergul Y, Ozyilmaz I,etal. Transcatheter closure of perimembranous ventricular septal defects usingNit-Occlud(®) Lê VSD coil: early and mid-term results. Pediatr Cardiol.2014;35(5):817-23. doi:10.1007/s00246-013-0860-8.
6. Xing Q, Pan S, An Q, Zhang Z, Li J, Li F,et al. Minimally invasiveperventricular device closure of perimembranous ventricular septal defectwithout cardiopulmonary bypass: multicenter experience and mid-term follow-up. JThorac Cardiovasc Surg. 2010;139(6):1409-15.doi:10.1016/j.jtcvs.2010.01.018.
7. Chen Q, Cao H, Zhang GC, Chen LW, Li QZ, Qiu ZH. Closure ofperimembranous ventricular septal defects with intraoperative device technique:another safe alternative to surgical repair. Thorac Cardiovasc Surg.2013;61(4):293-9. doi:10.1055/s-0032-1311532.
8. Amin Z, Danford DA, Lof J, Duncan KF, Froemming S. Intraoperativedevice closure of perimembranous ventricular septal defects withoutcardiopulmonary bypass: preliminary results with the perventricular technique. JThorac Cardiovasc Surg. 2004;127(1):234-41.doi:10.1016/j.jtcvs.2003.08.023.
9. Li G, Su J, Fan X, Li Z, Zhang J, Zhu Y, et al. Safety and efficacyof ventricular septal defect repair using a cosmetic shorter right lateralthoracotomy on infants weighing less than 5 kg. Heart Lung Circ.2015;24(9):898-904. doi:10.1016/j.hlc.2015.02.010. [MedLine]
10. Ma ZS, Dong MF, Yin QY, Feng ZY, Wang LX. Totally thoracoscopicrepair of ventricular septal defect: a short-term clinical observation on safetyand feasibility. J Thorac Cardiovasc Surg. 2011;142(4):850-4.doi:10.1016/j.jtcvs.2011.03.001.
11. Mongeon FP, Burkhart HM, Ammash NM, Dearani JA, Li Z, Warnes CA, etal. Indications and outcomes of surgical closure of ventricular septal defect inadults. JACC Cardiovasc Interv. 2010;3(3):290-7.doi:10.1016/j.jcin.2009.12.007.
12. Scully BB, Morales DL, Zafar F, McKenzie ED, Fraser CD Jr, HeinleJS. Current expectations for surgical repair of isolated ventricular septaldefects. Ann Thorac Surg. 2010;89(2):544-9; discussion 550-1.doi:10.1016/j.athoracsur.2009.10.057.
13. El-Sisi A, Sobhy R, Jaccoub V, Hamza H. Perimembranous ventricularseptal defect device closure: choosing between amplatzer duct occluder I and II.Pediatr Cardiol. 2017;38(3):596-602.doi:10.1007/s00246-016-1553-x. [MedLine]
14. Yang L, Tai BC, Khin LW, Quek SC. A systematic review on theefficacy and safety of transcatheter device closure of ventricular septaldefects (VSD). J Interv Cardiol. 2014;27(3):260-72.doi:10.1111/joic.12121.
15. Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG,et al. Transcatheter Closure of Perimembranous Ventricular Septal Defects. Earlyand Long-Term Results. J Am Coll Cardiol. 2007;50(12):1189-95.doi:10.1016/j.jacc.2007.03.068.
16. Ou-Yang WB, Wang SZ, Hu SS, Zhang FW, Zhang DW, Liu Y, et al.Perventricular device closure of perimembranous ventricular septal defect:effectiveness of symmetric and asymmetric occluders. Eur J Cardiothorac Surg.2017;51(3):478-482. doi:10.1093/ejcts/ezw352. [MedLine]
17. Zhu D, Gan C, Li X, An Q, Luo S, Tang H, et al. Perventriculardevice closure of perimembranous ventricular septal defect in pediatricpatients: technical and morphological considerations. Thorac Cardiovasc Surg.2013;61(4):300-6. doi:10.1055/s-0033-1334997.
18. Yang R, Sheng Y, Cao K, Kong X, Xu D, Yong Y, et al. Transcatheterclosure of perimembranous ventricular septal defect in children: safety andefficiency with symmetric and asymmetric occluders. Catheter Cardiovasc Interv.2011;77(1):84-90. doi:10.1002/ccd.22644. [MedLine]
19. Yang Y, Gao L, Xu X, Zhao T, Yang J, Gao Z, et al. Echocardiographicassessment and guidance in minimally invasive surgical device closure ofperimembranous ventricular septal defects. Heart Surg Forum. 2014;17(4):E206-11.doi:10.1532/HSF98.2014340.
20. Zhang GC, Chen Q, Chen LW, Cao H, Yang LP, Wu XJ,et al.Transthoracic echocardiographic guidance of minimally invasive perventriculardevice closure of perimembranous ventricular septal defect withoutcardiopulmonary bypass: initial experience. Eur Heart J Cardiovasc Imaging.2012;13(9):739-44. doi:10.1093/ehjci/jes028.
21. Walsh MA, Bialkowski J, Szkutnik M, Pawelec-Wojtalik M, Bobkowski W,Walsh KP. Atrioventricular block after transcatheter closure of perimembranousventricular septal defects. Heart. 2006;92(9):1295-7.doi:10.1136/hrt.2005.084988.
22. Butera G, Chessa M, Carminati M. Late complete atriovenous blockafter percutaneous closure of a perimembranous ventricular septal defect.Catheter Cardiovasc Interv. 2006;67(6):938-41.doi:10.1002/ccd.20696.
23. Zhou T, Shen XQ, Zhou SH, Fang ZF, Hu XQ, Zhao YS, et al.Atrioventricular block: a serious complication in and after transcatheterclosure of perimembranous ventricular septal defects. Clin Cardiol.2008;31(8):368-71. doi:10.1002/clc.20243.
Disclosure of grant(s) or other funding: This research was sponsored by Chinese national and Fujian provincial key clinical specialty construction programs.
No conflict of interest.
Authors' roles & responsibilities
XSH Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published
ZRL Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published
QC Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published
LSY Drafting the work or revising it critically for important intellectual content; final approval of the version to be published
HC Drafting the work or revising it critically for important intellectual content; final approval of the version to be published
LWC Drafting the work or revising it critically for important intellectual content; final approval of the version to be published
GCZ Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published
Article receive on Tuesday, October 30, 2018
Article accepted on Saturday, February 9, 2019
 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license