ABSTRACT
Objective: To develop an easy-handling totally closed pediatric peritoneal dialysis system and assess the sterility assurance level. Methods: From February to December 2008 was designed and developed a closed-system pediatric peritoneal dialysis at the Bioengineering Division of Braile Biomédica Indústria, Comércio e Representações S/A®. Twenty systems were manufactured and submitted to sterility assurance level testing, and were divided into Group A (10) - using the sterility test - and B (10) - ethylene oxide gas penetration. Results: In Group A, the sterility test was negative for bacteria and fungi proliferation within 14 days in all systems. In Group B, the gas penetration test showed that there was gas penetration in all points assessed. Conclusions: It was possible to develop a new easy-handling closed-system pediatric peritoneal dialysis and ensure its sterility.
RESUMO
OBJETIVO: Desenvolver um sistema de diálise peritoneal pediátrico totalmente fechado, de fácil manejo e avaliar o nível de garantia de esterilidade. MÉTODOS: No período de fevereiro a dezembro de 2008, foi idealizado e desenvolvido junto ao Setor de Bioengenharia da Braile Biomédica Indústria, Comércio e Representações S/A
® um sistema de diálise peritoneal pediátrico fechado. Foram construídos 20 sistemas submetidos a testes quanto à garantia do nível de esterilidade, sendo divididos em grupo A (n=10), utilizando-se teste de esterilidade, e B (n=10), penetração do gás óxido de etileno. RESULTADOS: No grupo A, o teste de esterilidade foi negativo para a proliferação de bactérias e fungos em 14 dias, em todos os sistemas. No grupo B, o teste de penetração de gás demonstrou que houve penetração de gás em todos os pontos avaliados. CONCLUSÃO: Foi possível desenvolver um novo sistema de diálise peritoneal pediátrico fechado de fácil manuseio e garantir sua esterilização.
INTRODUCTION
Cardiac surgery using cardiopulmonary bypass (CPB) represented one of the great medical achievements of the twentieth century, however, one of the side effects after its use is renal failure, which can occur due to many factors and are often more pronounced in children during newborn period [1,2].
When a child undergo correction of congenital heart defect with CPB and evolves with renal failure, this patient can be treated by peritoneal dialysis [3,4]. During dialysis, the solution is injected into the abdomen using a peritoneal catheter inserted in the region and, after a certain time, the liquid is drained thus restarting a new cycle [5,6].
Peritonitis is a frequent complication in patients on peritoneal dialysis and remains an important cause of morbidity and abandonment of the technique in patients under treatment [7,8]. The risk for the development of peritonitis due to several factors, such as prolonged time of treatment, rate of replacement of bathing solution, solution concentrations and use of the catheter, which maintains the communication of the peritoneum with the external environment [8].
Although automated techniques for peritoneal dialysis already exist, the manual technique is widely used in hospitals in developing countries due to the simplicity of handling of the replacement of bathing solution and smoothness of the process [9,10].
For the procedure, there is available in Brazil only a system of newborn peritoneal dialysis capable of controlling the volume of liquid to be infused that requires specific adapters for use [11]. In most services the system of peritoneal dialysis is performed manually by the connection of various independent circuits, designed for other purposes. This handling facilitates the occurrence of failures in the assembly with consequent increased risk of infection, particularly peritonitis [8].
With this concern, the aim of this study was to develop an easy-handling totally closed pediatric peritoneal dialysis system and assess the sterility assurance level.
METHODS
From February to December 2008 a totally closed pediatric peritoneal dialysis system was developed and tested in partnership with the Department of Bioengineering of Braile Biomédica Indústria, Comércio e Representações S/A®.
The closed system consists of two routes - one, for infusion of solution and another for drainage - with only two connections to establish the peritoneal dialysis (Figure 1). It consists of tubes, connectors and reservoirs made of polyvinyl chloride (PVC), connected and glued with solvent cyclohexanone.
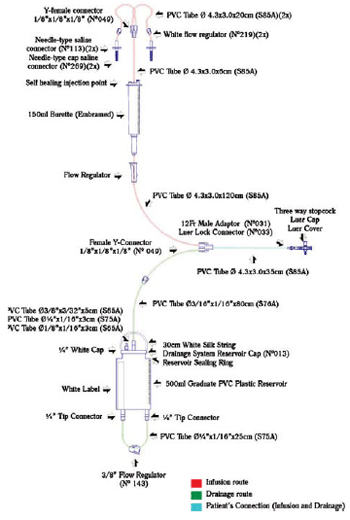
Fig. 1 - Scheme of the closed pediatric peritoneal dialysis system. The components and their respective sizes are shown. PVC- Polyvinyl chloride
20 pediatric peritoneal dialysis systems were mounted and packaged separately in plastic packaging and double cardboard box. The systems were sterilized in ethylene oxide gas (ETO) at a concentration of 592 mg, vacuum pressure of - 0.65 kg/cm
2 and pressure of + 0.42 kg/cm
2 for 4 hours. After sterilization, the systems were divided into two groups according to the test applied. Groups A, 10 systems submitted to the sterility test. Group B, 10 systems submitted to test of gas penetration.
The sterility test was performed at the Microbiology Laboratory, in classified area and consisted of washing of the whole system using 120 ml of 0.9% saline solution which then was collected in the infusion (60 ml) and drainage (60 ml) outlets. This solution was filtered on 0.45µm membrane and incubated in two different culture media - one for bacteria and another for fungi. For analysis and reading of the results, the systems were sequentially identified from S1 to S10. As positive control, the culture media were submitted to the growth promotion test (Growth Promotion Test), with inoculation of strains.
The gas penetration test (Group B) was performed by means of chemical indicators distributed in the system as shown in Figure 2. After sterilization the 10 systems have been destroyed for removal of indicators and identified sequentially as S11.1 to S20.5.
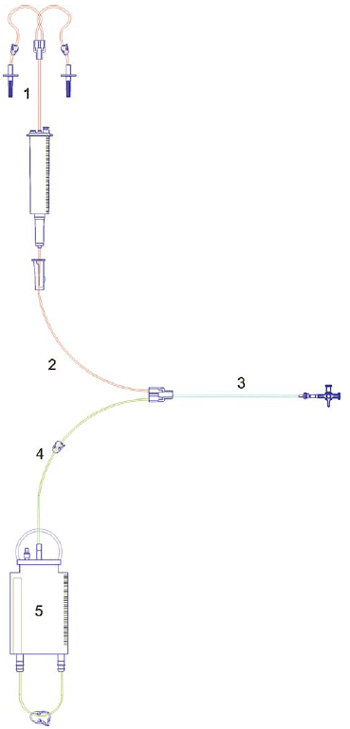
Fig. 2 - Scheme of the closed pediatric peritoneal dialysis system showing places on which the chemical indicators were inserted
The result of the sterility test (Group A) of all membranes was negative for bacteria and fungi.
In the test of ETO gas penetration (Group B) was observed change of color in all chemical indicators.
DISCUSSION
The new system of closed pediatric peritoneal dialysis, which requires minimal handling, may reduce the risk of peritonitis when applied to patients. In literature there are studies showing increase in the rate of abdominal infection in patients using open systems [5, 12]. In the study by Mott et al [12], assessing the number of contamination and risk of infectious complications during peritoneal dialysis, it was observed that 62% of patients who had undergone dialysis sessions using open systems presented infections against 23.3% in closed systems.
The system developed is composed of graduate collector and serum dripper of micro-drops type, used to control the volume of infusion of the dialysis solution to the abdomen, since the infusion of fluid during the procedure causes increased abdominal pressure, leading to impact on cardiorespiratory system, particularly in patients with low cardiac output [13].
Self healing injection points used for injections of drugs (Figure 1) allow accomplishment of electrolytes corrections when necessary because it is known that during treatment the child may present metabolic disorders [14].
The three-way stopcock used to attach the dialysis system to the catheter is of universal type, or that is, it connects to any type of dialysis catheter, thus exempting the use of adapters - used in other systems [11]. One way of the stopcock can be used for collection of peritoneal fluid or to install the monitoring system of intra-abdominal pressure (IAP). The measurement of IAP is important to assess the tolerance of patient's infusion, and it is recommended that the pressure should maintained between 5 and 15 cmH
2O [13].
The 500 ml graduated drainage reservoir allows the storage of larger volumes without the need for frequent discard. The hydrophobic air filter in the upper portion of the reservoir prevents contamination of the reservoir by air. The PVC tube that drains the fluid is protected by a safety cap for sterilization and removal of the system from the packaging without contaminating the distal route. The blind-type tip connector should be used to connect the PVC tube to the reservoir in case of loss of the safety cap, by ensuring greater protection to the system.
For the safe clinical use of peritoneal dialysis systems is crucial to ensure sterilization of the product [15]. The effectiveness of the sterilization was confirmed by indirect inoculation sterility tests. In order to provide validity to the test, the quality of the environment on which the test is performed - as well as the culture media, temperature and time of incubation - are important factors. In cases of sterilization by ETO, the biological and chemical indicators show the diffusion and penetration of the gas inside the material, especially when it deals with complex plastic materials and multiple packagings [16]. The gas is an effective sterilizing agent used in thermosensible articles and presents good penetration into packaging and lumens. Its lethal action is attributed to alkylation of microbial protein chains, avoiding the cell proliferation [15].
The development of this new closed pediatric peritoneal dialysis system opens perspectives for clinical studies in order to assess possible future practical applications.
CONCLUSIONS
It was possible to develop a new easy-handling totally closed pediatric peritoneal dialysis system and ensure its sterilization, suggesting its future clinical application.
REFERENCES
1. Souza MH, Elias DO. Fundamentos da circulação extracorpórea. 2ª ed. Rio de Janeiro:Centro Editorial Alfa Rio; 2006.
2. Croti UA, Braile DM, Beani L, Fleury MCP. Monocúspide de homoenxerto decelularizado no tratamento do truncus arteriosus com a técnica de Barbero Marcial. Rev Bras Cir Cardiovasc. 2008;23(2):290-1. [MedLine] View article
3. Elias DO, Souza MHL, Lacerda BS, Fagundes FES, Lino FJS, Tiraboshi M. Injúria orgânica da circulação extracorpórea nos três primeiros meses de vida. Rev Bras Cir Cardiovasc. 1990;5(1):1-8. View article
4. Utley JR. Renal effects of cardiopulmonary bypass. In Utley JR. Pathophysiology and techniques of cardiopulmonary bypass. Baltimore:Williams & Wilkins;1982.
5. Guimarães RAC. Análise da implantação do sistema fechado simplificado durante a assistência de enfermagem aos clientes em diálise peritoneal intermitente manual. Rev Med Aeronaut Bras. 1993;43(1/2):10-9.
6. Calderón Elvir C, Duarte V, Juan C, Maza Vallejo J, Peralta Bustamante A. Uso y manejo del catéter de diálisis peritoneal permanente en pediatría. Acta Pediátr Méx. 1996;17(2):64-6.
7. Lima L, Mota C, Lira S, Faria M, Costa T, Pereira E. Peritonites em diálise peritoneal pediátrica. Rev Port Nefrol Hipert. 2005;19(2):103-10.
8. Noblat ACB, Mello ME, Leite EB, Luna MAC, Almeida ARP, Martinelli R. Peritonite por "B. subtilis" em diálise peritoneal intermitente, crônica. J Bras Nefrol. 1988;10(3):99-102.
9. Castro RP, Croti UA, Machado MN, Murillo HG, Rincon OYP, Policarpo SR, et al. Ultrafiltração convencional com modificação técnica no tratamento cirúrgico dos defeitos cardíacos congênitos. Rev Bras Cir Cardiovasc. 2006;21(1):42-9. View article
10. Cavagnaro FSM. Terapias continuas de reemplazo renal agudo en pediatría. Rev Chil Urol. 2003;68(2):125-30.
11. Fresenius medical care. Accessed in 21/01/2009. Avaliable from: URL: http://www.fmc-ag.com.br
12. Motti EF, Hutzler RU, Granato CF, Giorgi DM, Burdmann EA, Rodrigues E, et al. Contaminação bacteriana em diálise peritoneal, com sistema de drenagem aberto e fechado. J Bras Nefrol. 1982;4(3/4):69-72.
13. Barcellos PG, Johnston C, Carvalho WB, Fonseca MC, Santos JE, Bandini E. Repercussões cardiorrespiratórias da diálise peritoneal em crianças graves. RBTI. 2008;20(1):31-6.
14. Abensur H. Uso da diálise peritoneal em pacientes com insuficiência cardíaca congestiva. Rev Bras Hipertens. 2008;15(3):162-5.
15. Ramos EMN, Costa MFR, Oliveira OC, Ikeda T, Guimarães ZS. Central de material e esterilização. Manual técnico. Brasília; 2000.
16. Pinto TJA, Kaneko TM, Ohara MT. Controle biológico de qualidade de produtos farmacêuticos correlatos e cosméticos. 2ª ed. São Paulo: Atheneu; 2003. p.153-78.
CONFLICT OF INTEREST STATEMENT:
The authors declare that they have conflict of interests because the study was developed and partially funded by Braile Biomédica® company.
Article receive on Friday, January 2, 2009


 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license