Görkem YiğitI; Anıl ÖzenI; Ferit ÇetinkayaI; Ertekin Utku UnalI; Hakki Zafer IscanI; Cemal Levent BirincioğluI; Ahmet SarıtaşI
DOI: 10.21470/1678-9741-2020-0133
ABSTRACT
Introduction: Valve-reimplantation and remodelling techniques used in aortic reconstruction provide successful early, mid, and long-term results. We present our early and late-term experience with 110 patients with aortic regurgitation (AR) who underwent aortic valve repair (AVr) or valve-sparing aortic root surgeries (VSARS) due to aortic dissection or aortic aneurysm.AR = Aortic regurgitation
ASCP = Antegrade selective cerebral perfusion
AVr = Aortic valve repair
AVR = Aortic valve replacement
BAV = Bicuspid aortic valve
CHF = Chronic heart failure
COPD = Chronic obstructive pulmonary disease
CPB = Cardiopulmonary bypass
CVA = Cerebrovascular accident
EF = Ejection fraction
INR = International Normalization Ratio
LVEDD = Left ventricular end-diastolic diameter
MFS = Marfan syndrome
NYH = New York Heart Association
SCG = Supracoronary graft replacement
SPSS = Statistical Package for the Social Sciences
STJ = Sinotubular junction
VSARS = Valve-sparing aortic root surgery
XCL = X-clamp
INTRODUCTION
Aortic valve reconstruction techniques have been available since the late 1950s. However, poor surgical outcomes resulted in aortic valve replacement (AVR) being predominantly preferred until the 1990s. In the early 1990s, aortic valve-sparing operations were initiated under the leadership of David and Yacoub and became widespread in the light of their long-term successful results[1]. Valve-reimplantation and remodelling techniques used in aortic reconstruction provide successful early, mid, and long-term results when applied to appropriate patients by an experienced surgical team.
Valve-protective surgery aims to preserve the patient's native valve and prevent prosthetic valve replacement surgery. In patients undergoing mechanical valve replacement, catastrophic complications such as valvular thrombosis and mechanical valve dysfunction due to ineffective coumadin use may occur. Furthermore, bleeding due to high International Normalization Ratio (INR) values and prosthetic valve endocarditis are amongst other significant complications. In addition, the possibility of life-threatening conditions and complications related to mechanical valve replacement, such as patient-prosthetic valve mismatch, pannus, and paravalvular leak, require the use of valve repair and valve-sparing surgery[2].
The aim of our study was to determine early and late survival, degree of postoperative aortic regurgitation (AR), the incidence of redo cases, and early and late postoperative complication rates in patients diagnosed with aortic aneurysm or aortic dissection with AR undergoing aortic valve repair (AVr) or valve-sparing aortic root surgery (VSARS).
METHODS
Study Population
Nine hundred eighty-two patients who underwent aortic valve surgery and aortic aneurysm or dissection surgery between April 1997 and January 2017 were analysed using the patient database (Sarus and Avicenna automation systems) and examined by scanning files from hospital archives. A total of 110 patients with AR who underwent AVr or VSARS due to aortic dissection or aortic aneurysm were included in the study. Patients with AVR and patients without intervention of the aortic valve were excluded from the study.
There were only two mortalities in 110 patients (one intraoperatively and one at the fifth postoperative hour). Hence, a total of 108 patients were followed up. Preoperative data regarding age, sex, presence of Marfan syndrome (MFS), echocardiographic findings (aneurysm diameter, AR or stenosis degree, left ventricular end-diastolic diameter [LVEDD], ejection fraction [EF]), and data about other valve pathologies were obtained.
Intraoperative data regarding x-clamp (XCL) time, cardiopulmonary bypass (CPB) time, antegrade selective cerebral perfusion (ASCP) time, cooling degree, type of operation, need for inotropic support, and operative mortality data were collected.
In the postoperative period, early and late survival, mortality and morbidity rates, echocardiographic findings (AR or stenosis degree, LVEDDs, EF), incidence of being a redo case, causes of early and late mortality and morbidity, and postoperative complications were investigated. The minimum follow-up period was two months and the longest one was 108 months.
Echocardiographic Assessment
All patients underwent post-repair intraoperative transepicardial or transoesophageal echocardiographic analysis. Transthoracic echocardiography was performed for all patients prior to discharge and at regular intervals for living patients with native valve during the course of follow-up.
Statistical Analysis
All data were analysed using the Statistical Package for the Social Sciences (SPSS) software (SPSS Inc., Chicago, United States of America), version 15.0. The normal distribution of the variables was evaluated visually, using histograms and probability graphs, and analytically, using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Normally distributed continuous variables were expressed in means and standard deviation whereas non-normally distributed continuous variables were presented using median and interquartile range values. Data on categorical variables were expressed in numbers and percentages. Preopereative and postoperative data was analysed using the Wilcoxon test. The Kaplan-Meier was used to evaluate freedom from medium-severe AR and freedom from reoperation. A different log-rank analysis was preferred to study the effect of valve-sparing surgery on survival. P-values < 0.05 were considered statistically significant.
RESULTS
A total of 108 patients with AR who underwent AVr or VSARS due to aortic dissection or aortic aneurysm were included in the study. Twenty of these patients had aortic dissection (18.5%), seven (6.4%) had MFS, and five (4.6%) had bicuspid aortic valve (BAV) (Table 1).
| Sex (N=108) | Female | 36 (33.3%) |
| Male | 72 (66.7%) | |
| Age (years) | 57.25±13.20 (20-82) | |
| Hypertension (N=108) | 72 (66.6%) | |
| Diabetes mellitus (N=108) | 12 (11.1%) | |
| Hyperlipidemia (N=108) | 15 (13.8%) | |
| History of CVA (N=108) | 6 (5.5%) | |
| Chronic kidney disease (N=108) | 1 (0.9%) | |
| COPD (N=108) | 5 (4.6%) | |
| Marfan syndrome (N=108) | 7 (6.4%) | |
| CHF NYHA class (N=108) |
Class I | 37 (34.3%) |
| Class II | 34 (31.5%) | |
| Class III | 28 (25.9%) | |
| Class IV | 9 (8.3%) | |
| Rhythm (N=108) |
Sinus rhythm | 106 (98.1%) |
| Atrial fibrillation | 2 (1.8%) | |
| Operation type (N=108) |
Urgent | 20 (18.5%) |
| Elective | 88 (81.5%) | |
| Patients' diagnosis (N=108) | Type A aortic dissection | 20 (18.5%) |
| Aortic regurgitation and ascending aortic aneurysm | 84 (77.7%) | |
| Aortic regurgitation and ascending aortic aneurysm with arcus aortic aneurysm | 4 (3.7%) | |
| Bicuspid valve | 5 (4.6%) | |
| Operative procedure (N=108) | SCG + aortic valve intervention | 94 (87%) |
| Sinus remodelling + aortic valve intervention | 14 (13%) | |
| Additional surgical procedure performed on the aortic valve | Resuspension | 74 (68.5%) |
| Plication | 25 (23.1%) | |
| Commissurotomy | 9 (8.3%) | |
| Additional surgical
procedure (N=21) |
Coronary artery bypass grafting | 17 (15.7%) |
| Mitral valve replacement | 4 (3.7%) | |
| Total arcus replacement | 10 (9.2%) | |
The mean follow-up of our study was 25.29±24.81 (2-108) months. Seventy-two (66.7%) of the patients were male. There were only two mortalities out of 110 patients (one intraoperatively and one at the fifth postoperative hour). Hence, a total of 108 patients were followed up. Aortic valve intervention and supracoronary graft replacement (SCG) were performed in 94 patients (87%); aortic valve intervention and remodelling were performed in 14 patients (13%) (Table 1).
The mean age of the patients included in our study was 57.25±13.20 years. The youngest patient was a 24-year-old female with AR and ascending aortic aneurysm who underwent AVr and SCG. The oldest patient was an 82-year-old woman who underwent AVr, SCG, and two-vessel coronary bypass surgery for AR with coronary artery disease and ascending aortic aneurysm.
Mean operative time was 333.36±13.20 (180-780) minutes; mean CPB duration was 126.16±48.66 (54-352) minutes, and mean XCL duration was 82.28±32.24 (32-169) minutes. Only 42 patients (39.8%) underwent ASCP (Table 2).
| Operation time (min) | 333.36±13.20 (180-780) | |
| XCL time (min) | 82.28±32.24 (32-169) | |
| CPB time (min) | 126.16±48.66 (54-352) | |
| ASCP time (min) | 20.98±13.60 (8-68) | |
| ASCP (min) | Applied | 42 (38.9%) |
| Unapplied | 66 (61.1%) | |
| Cooling degree (ºC) | 27.74±2.03 (19-34) |
Postoperative data including the need for inotropic support, eryhrocyte suspension, and fresh frozen plasma are given in Table 3.
| Inotropic administration (N=108) | None | 37 (34.3%) |
| Single | 24 (22.2%) | |
| Two | 47 (43.5%) | |
| Drainage (cc) | 910.93±726.67 (50-4250) | |
| Intubation time (h) | 18.62±41.04 (5-400) | |
| Erythrocyte suspension transfusion (Unit) | 1.42±2.30 (1-18) | |
| Fresh frozen plasma transfusion (Unit) | 3.34±5.56 (2-50) |
In the postoperative period, 13 patients (12%) underwent revision for tamponade and six patients (5.6%) underwent revision for bleeding. Sixteen of these patients belonged to the SCG and AVr group, whereas three belonged to the remodelling and AVr group. Stroke developed in five patients (4.6%), pneumonia in one patient, mediastinitis in one patient (0.9%), and renal failure in one patient (0.9%). Eight patients (7.4%) underwent AVR during follow-up.
Eight patients (7.4%) underwent AVR during follow-up. One, two, and five-year freedom from reoperation were 97.9%, 93.6%, and 81%, respectively (Figure 1). Three patients who had undergone SCG and AVr with preoperative 2nd degree and postoperative 3rd degree of AR, underwent AVR at the 57th, 60th, and 64th postoperative months; two patients who had undergone SCG and AVr with preoperative 3rd to 4th and 3rd degree and postoperative 3rd to 4th degree of AR underwent AVR at the 20th and 24th postoperative months; one patient who had undergone Yacoub procedure with preoperative 1st degree and postoperative 3rd degree of AR underwent AVR at the 61th postoperative month; and two patients who had undergone SCG and AVr due to acute aortic dissection with preoperative 1st to 2nd degree and postoperative 3rd degree of AR underwent AVR at the 24th and 108th postoperative months (Table 4).
| Patient | Operation type | Preop. AR | Postop. AR | Follow-up period (month) |
|---|---|---|---|---|
| 1 | SCG and aortic valve plication | 2 | 3 | 57 |
| 2 | SCG and aortic valve resuspension | 2 | 3 | 60 |
| 3 | SCG and aortic valve resuspension (bicuspid) | 2 | 3 | 64 |
| 4 | SCG and aortic valve resuspension | 3/4 | 3/4 | 20 |
| 5 | SCG and aortic valve resuspension | 3 | 3/4 | 24 |
| 6 | Aortic remodelling and aortic valve plication | 1 | 3 | 61 |
| 7 | SCG and aortic valve resuspension (type 1 dissection) | 1/2 | 3 | 24 |
| 8 | SCG and aortic valve resuspension (type 1 dissection) | 1/2 | 3 | 108 |
When the AR, EF, and diastolic ventricular diameters were compared in the preoperative and postoperative period, the differences were statistically significant. In the postoperative period, a decrease was observed in AR compared to the preoperative period (P<.001); there was an increase in postoperative EF compared to preoperative values (P<.005) and significant decrease in postoperative left ventricle diameters compared to preoperative values (P<.001) (Table 5). Kaplan-Meier analysis revealed one, two, four, and five-year freedom from moderate-severe AR as 95%, 91%, 87%, and 70%, respectively (Figure 2).
| Preoperative | Postoperative | P-value | |
|---|---|---|---|
| AR | 2.05±0.61 (1-3.5) | 1.41±0.78 (0-3) | <0.001 |
| EF | 52.71±8.01 (20-65) | 54.45±7.49 (23-69) | <0.005 |
| LVEDD | 5.25±0.76 (4-8.2) | 4.97±0.64 (3.9-7.4) | <0.001 |
When the remodelling and non-remodelling groups were compared, no difference was found between the two methods in terms of freedom from AR (P=.832) (Figure 3).
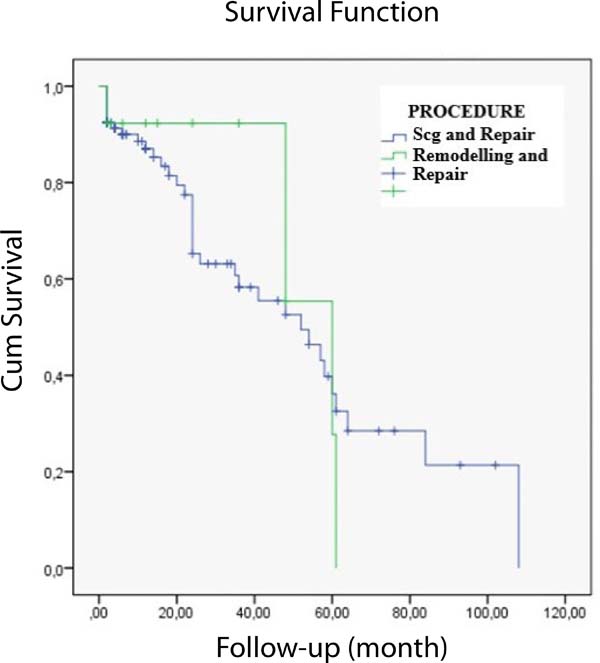
Out of 100 remaining patients, 13 (12%) had minimum AR, 52 (48%) had 1st-2nd degree AR, and 35 (32%) had 2nd-3rd degree AR during follow-up.
DISCUSSION
Aortic valve reconstruction techniques have been available since the late 1950s. However, poor surgical outcomes resulted in AVR being predominantly preferred until the 1990s. Valve-sparing operations gained accelaration after the 1990s due to high complication rates. Bleeding rates following mechanical valve replacement after 10 an 20 years are 16% and 61%, respectively[3], and thromboembolic complication rates after 10 and 20 years were shown as 10% and 24%, respectively[3]. In addition, another major issue for this patient population is the economical burden caused by INR follow-up.
Bearing all the aforementioned disadvantages, interest in valve-sparing operations are growing. Benthall and its modifications have been accepted as golden standart[1,4,5] for the surgical treatment of aortic root pathologies. Nevertheless, VSARS have become widely performed, due to successful long-term outcomes of these operations pioneered by David and Yacoub at the early 1990s[1]. Valve-sparing reimplantation and remodelling techniques used in aortic reconstruction possess successful short and long-term results, when applied to the appropriate patients by experienced surgical teams and should be considered primarily for patients with annuloaortic ectasia[2]. All the operations in this series were perfomed by several experienced surgeons and the early and long-term results are satisfactory.
There are few studies on isolated AVr in the literature. Freedom from reoperation rates were displayed as 95% in five years for AR resulting from prolapsus[6,7]. Aicher et al. shared their successful, long-term follow-up results of 15 years. They performed isolated AVr in 1083 patients between 1999 and 2015; 583 (54%) of these patients had tricuspid valves. Freedom from reintervention for tricuspid valves and bicuspid valves in five, 10, and 15 years were 94% and 84%, 81% and 90%, 78% and 71%, respectively[6]. This result reveals that success of the repair of tricuspid valves is higher than that of the bicuspid ones. Furthermore, presence of enlarged annulus or sinotubular junction (STJ) in their series was one of the main risk factors for failed valve repair. Combining annulus reduction or STJ remodelling with AVr have improved the long-term results[8,9].
VSARS is the choice of treatment for aortic root disease with functional leaflets, even though AR accompanies the pathology. However, AR is not always due to root dilatation and may require a combined treatment using cusp repair. Significant preoperative AR and cusp repair seem to be risk factors for poor prognosis following VSARS[10]. Nonetheless, Schafers et al.[11] applied an aggressive approach of combined leaflet prolapsus repair with VSARS and found no significance in operating times, mortality, and survival[11,12]. Besides this, Schafers preferred suture annuloplasy and Lansac used external ring annuluplasty in addition to reimplantation in order to stabilize the aortic root[13].
In light of these developments, we retrospectively studied 108 patients, diagnosed with AR due to aortic aneurysm or dissection who underwent AVr or VSARS.
VSARS is usually preffered in young patients diagnosed with MFS and bicuspid aorta, aiming to stabilize the aortic annulus[14-16]. This study included 6.4% MFS patients and 4.6% congenital/bicuspid valve patients. The mean age of the study participants was 57 years and they can be considered as relatively young.
Preservation of cuspis geometry is the most important element of valve-sparing surgery. Together with minimal central AR, the final aim at the end of surgery should be no cuspis prolapsus and a coaptation height above the nadir of the aortic annulus.
There is a limited number of studies in the literature regarding the long-term outcome of patients undergoing VSARS. The ratios for survival after five and 10 years are 85-98.7% and 70-93.5%, respectively[17-20]. No mortalities occured in our series.
Acute aortic dissection is a significant risk factor for early mortality following VSARS[18,21]. Nevertheless, there were no mortalities amongst the patients who underwent surgery due to acute aortic dissection in our study. In contrast, the study of Shresta et al.[18] revealed six early deaths (four patients with diagnosis of aortic dissection) out of 126 patients. The total number of patients undergoing surgery due to acute aortic dissection was 21 in their study. Twenty (18.5%) patients were operated with a diagnosis of acute type 1 aortic dissection in our study. All of these patients underwent SCG, AVR, and additional procedures. None of them underwent remodelling operations. There is no mortality during early, mid, and long-term follow-up of these patients. This may be explained by the fact that our patients with aortic dissection were not critically ill, malperfused, or comorbid patients as in the other mentioned studies.
Valve-sparing procedures are complex procedures requiring prolonged duration of XCL and CPB that may result in coagulopathies. Hence, the most common complication following such procedures is postoperative bleeding[21]. In the present study, following surgery, 13 (12%) patients developed tamponade and six (5.6%) patients underwent exploration due to bleeding. Five patients (4.6%) developed cerebrovascular accident, one (0.9%) had pneumonia, one (0.9%) had mediastinitis, and one (0.9%) had chronic renal failure.
The most significant problem after aortic valve-sparing and AVr procedures is AR and the need for consequent reoperation. Development of early AR following surgery is frequently due to technical failure[22]. None of the patients in the present study developed early AR. However, development of late AR mainly results from cusp degeneration and aortic root dilatation. David et al.[23] announced five, 10, and 15-year freedom from mid-severe AR rates as 98.3%±3.5%, 92.9%±6.5%, and 89.4%±12%, respectively, in their study involving 296 patients. Furthermore, Coselli et al.[24] shared their experience of 83 patients in 2014. They revealed two, four, six, and eight-year freedom from mid-severe AR rates as 94.8%±2.6%, 81.1%±5.3%, 77.8%±6%, and 73.9%±6.9%, respectively. The findings of the present study report the results of a maximum five-year period, evaluated by Kaplan-Meier analysis. Freedom from mid-severe AR rates are 95%, 91%, 87%, and 70% in one, two, four, and five years, respectively, and the result of freedom from AR in five years is similar to the one of Coselli et al.[24]
Eight patients in the present study underwent AVR during the follow-up period; one patient at the postoperative 20th month and two patients at the postoperative 24th month. Consequently, these three patients underwent AVR approximately at the postoperative second year. Four patients underwent AVR at the postoperative 57th, 60th, 61st, and 64th months, which means that they underwent valve replacement approximately at the postoperative fifth year. Finally, one patient underwent AVR at the postoperative 108th month, which is nine years following SCG and AVr due to acute type 1 aortic dissection. Annular stabilization was performed only in a single 73-year-old male patient, who had undergone AVr and remodelling due to AR and ascending aortic aneurysm. His preoperative echocardiography reported 1st degree AR and postoperative echocardiography reported 3rd degree AR. He underwent AVR at the postoperative 61th month. None of the patients undergoing AVR had annular dilatation. According to the operation notes, the cause of valve replacement was cusp degeneration. The rate of freedom from reoperation in 10 years is 81-98% in the literature[25]. Leipzig group stated the five-year freedom from reoperation rate as 95.9%[26]. In addition, six patients out of 233 underwent AVR in the study of Kvitting et al.[19] published in 2013. Their freedom from reoperation rates in five and 10 years were 98.0%±1.2% and 92.2%±3.6%, respectively. In another study, David et al.[27] (2013) reported that seven patients underwent reoperation out of 374 patients. Freedom from reoperation in their report in 10, 15, and 20 years were 97.1%, 94.2%, and 94.2%, respectively. Our study revealed freedom from reoperation in one, two, and five years as 97.9%, 93.6%, and 81%, respectively. This result displays lower rates of freedom from reintervention compared to the aforementioned studies and maybe a result of presence of lower number of patients compared to other studies since our institute started performing valve-sparing operations 10 years later than the pioneering centers. Besides this, annular stabilization was applied only in a few patients and the operations were perfomed by six different surgeons.
Surgical approach for bicuspid valves is a topic much debated in the literature. Studies reveal worse outcomes for BAV compared to tricuspid ones, following valve-sparing operations[28]. A recent study by Shrestha et al.[29] found high reoperation rate for patients with BAV (25%) with 7.2±4.7 years of follow-up. Their study revealed freedom from reoperation for patients with BAV as 68% in 10 years. However, Schafers et al.[30] displayed freedom from reoperation in five years as 97%, for 173 patients undergoing AVr. Furthermore, the same surgical team published a more recent updated study including 316 patients demonstrating survival and freedom from reoperation rates in 10 years as 92% and 81%, respectively[30,31]. In our study, there were only five patients with BAV. Three of these patients underwent SCG and aortic valve resuspension, one underwent SCG and aortic valve commisurotomy, and one underwent Yacoub remodelling and aortic valve resuspension. A 43-year-old male patient with SCG and aortic valve resuspension underwent AVR at the 64th postoperative month. The etiology of AR was cuspis prolapsus according to the operating note.
Another disputed subject is MFS. Martens et al.[32] operated 104 patients with MFS by VSARS. They achieved 86% freedom from reoperation in 10 years and 80% freedom from reoperation in 20 years. David procedure is recommended instead of Yacoub procedure for patients with MFS, in order to provide annular stabilization and prevent annular dilatation[33,34]. In our study, there were seven patients with MFS. Only one patient underwent AVR at the 108th postoperative month. This patient was a 48-year-old male who had undergone urgent surgery due to acute type 1 aortic dissection. Aortic valve resuspension and SCG replacement had been performed as an operation. The preoperative echocardiography revealed 1st-2nd degree AR with an LVEDD of 5.8 cm. He became symptomatic with 3rd degree of AR and an LVEDD of 7 cm at the postoperative nineth year and underwent AVR. Considering that his reoperation took place at the age of 57 years, a nine-year period without a mechanical valve and its disadvantages makes the decision to perform valve-sparing surgery during the first operation reasonable.
A significant finding of our study following aortic valve-sparing surgery, is the favorable values when comparing the preoperative and postoperative EF, LVEDD, and AR data. Monsefi et al.[35] did not find any statistical difference between the preoperative and postoperative EF and LVEDD values. Nevertheless, there was an increase in postoperative EF compared to preoperative values and significant decrease in postoperative LVEDD compared to preoperative values in our study. Furthermore, in the postoperative period, a decrease was observed in AR compared to the preoperative period in the present study.
Limitations
The retrospective nature of the study and shorter follow-up period compared to other large series are the main limitations of our study. Lower number of patients compared to other studies may be the main reason for no mortality and lower complication rates in the present study. Furthermore, application of annular stabilization in a limited number of patients is another limitation.
CONCLUSION
AVr and valve-sparing procedures have become an alternative for valve replacement surgery for suitable patients in the last two decades. It is essential that these procedures have also started to appear in the current guidelines. Better understanding of the underlying pathology together with current advances in surgical techniques and long-term follow-up studies in the literature are going to allow for better results to be obtained. According to the European Society of Cardiology 2017 Guidelines, performing valve repair and valve-sparing procedures in young patients with aortic root dilatation is a class I indication. When we take a look at our last 20 years of experience in aortic valve-sparing procedures, there was no mortality during follow-up and the rates of freedom from reoperation in one, two, and five years were 97.9%, 93.6%, and 81%, respectively. In addition, freedom from mid-severe AR in five years was 70%. Only eight (7.4%) patients underwent AVR during follow-up. In conclusion, when considering the favourable postoperative echocardiographic findings, we believe that one should perform valve-sparing procedures for appropriate patients.
REFERENCES
1. Bentall H, De Bono A. A technique for complete replacement of the ascending aorta. Thorax. 1968;23(4):338-9. doi:10.1136/thx.23.4.338.
2. Misawa Y. Valve-related complications after mechanical heart valve implantation. Surg Today. 2015;45(10):1205-9. doi:10.1007/s00595-014-1104-0.
3. Oxenham H, Bloomfield P, Wheatley DJ, Lee RJ, Cunningham J, Prescott RJ, et al. Twenty year comparison of a Bjork-Shiley mechanical heart valve with porcine bioprostheses. Heart. 2003;89(7):715-21. doi:10.1136/heart.89.7.715.
4. Cabrol C, Pavie A, Gandjbakhch I, Villemot JP, Guiraudon G, Laughlin L, et al. Complete replacement of the ascending aorta with reimplantation of the coronary arteries: new surgical approach. J Thorac Cardiovasc Surg. 1981;81(2):309-15. doi:10.1016/S0022-5223(19)37641-X.
5. Miller DC, Stinson EB, Oyer PE, Moreno-Cabral RJ, Reitz BA, Rossiter SJ, et al. Concomitant resection of ascending aortic aneurysm and replacement of the aortic valve: operative and long-term results with “conventional” techniques in ninety patients. J Thorac Cardiovasc Surg. 1980;79(3):388-401. doi:10.1016/S0022-5223(19)37948-6. [MedLine]
6. Aicher D, Langer F, Adam O, Tscholl D, Lausberg H, Schäfers HJ. Cusp repair in aortic valve reconstruction: does the technique affect stability? J Thorac Cardiovasc Surg. 2007;134(6):1533-8; discussion 1538-9. doi:10.1016/j.jtcvs.2007.08.023.
7. de Kerchove L, Boodhwani M, Glineur D, Poncelet A, Rubay J, Watremez C, et al. Cusp prolapse repair in trileaflet aortic valves: free margin plication and free margin resuspension techniques. Ann Thorac Surg. 2009;88(2):455-61; discussion 461. doi:10.1016/j.athoracsur.2009.04.064.
8. Asano M, Kunihara T, Aicher D, El Beyrouti H, Rodionycheva S, Schäfers HJ. Mid-term results after sinutubular junction remodelling with aortic cusp repair. Eur J Cardiothorac Surg. 2012;42(6):1010-5. doi:10.1093/ejcts/ezs120.
9. Lansac E, Di Centa I, Sleilaty G, Lejeune S, Khelil N, Berrebi A, et al. Long-term results of external aortic ring annuloplasty for aortic valve repair. Eur J Cardiothorac Surg. 2016;50(2):350-60. doi:10.1093/ejcts/ezw070.
10. Settepani F, Bergonzini M, Barbone A, Citterio E, Basciu A, Ornaghi D, et al. Reimplantation valve-sparing aortic root replacement with the Valsalva graft: what have we learnt after 100 cases? Interact Cardiovasc Thorac Surg. 2009;9(1):113-6. doi:10.1510/icvts.2009.202622.
11. Schäfers HJ, Aicher D, Langer F. Correction of leaflet prolapse in valve-preserving aortic replacement: pushing the limits? Ann Thorac Surg. 2002;74(5):S1762-4; discussion S1792-9. doi:10.1016/s0003-4975(02)04136-x.
12. Langer F, Aicher D, Kissinger A, Wendler O, Lausberg H, Fries R, et al. Aortic valve repair using a differentiated surgical strategy. Circulation. 2004;110(11 Suppl 1):II67-73. doi:10.1161/01.CIR.0000138383.01283.b8.
13. Aicher D, Schneider U, Schmied W, Kunihara T, Tochii M, Schäfers HJ. Early results with annular support in reconstruction of the bicuspid aortic valve. J Thorac Cardiovasc Surg. 2013;145(3 Suppl):S30-4. doi:10.1016/j.jtcvs.2012.11.059.
14. Lillehei CW. The society lecture. European society for cardiovascular surgery meeting, Montpellier, France, September 1992. The birth of open-heart surgery: then the golden years. Cardiovasc Surg. 1994;2(3):308-17. Erratum in: Cardiovasc Surg 1994;2(5):566. doi:10.1177/096721099400200303.
15. De Bakey ME, Cooley DA. Successful resection of aneurysm of thoracic aorta and replacement by graft. J Am Med Assoc. 1953;152(8):673-6. doi:10.1001/jama.1953.03690080017005.
16. Cooley DA, De Bakey ME. Resection of entire ascending aorta in fusiform aneurysm using cardiac bypass. J Am Med Assoc. 1956;162(12):1158-9. doi:10.1001/jama.1956.72970290003013a.
17. David TE, Feindel CM, Webb GD, Colman JM, Armstrong S, Maganti M. Long-term results of aortic valve-sparing operations for aortic root aneurysm. J Thorac Cardiovasc Surg. 2006;132(2):347-54. doi:10.1016/j.jtcvs.2006.03.053.
18. Shrestha M, Baraki H, Maeding I, Fitzner S, Sarikouch S, Khaladj N, et al. Long-term results after aortic valve-sparing operation (David I). Eur J Cardiothorac Surg. 2012;41(1):56-61; discussion 61-2. doi:10.1016/j.ejcts.2011.04.012.
19. Kvitting JP, Kari FA, Fischbein MP, Liang DH, Beraud AS, Stephens EH, et al. David valve-sparing aortic root replacement: equivalent mid-term outcome for different valve types with or without connective tissue disorder. J Thorac Cardiovasc Surg. 2013;145(1):117-26, 127.e1-5; discussion 126-7. doi:10.1016/j.jtcvs.2012.09.013.
20. Jeanmart H, de Kerchove L, Glineur D, Goffinet JM, Rougui I, Van Dyck M, et al. Aortic valve repair: the functional approach to leaflet prolapse and valve-sparing surgery. Ann Thorac Surg. 2007;83(2):S746-51; discussion S785-90. doi:10.1016/j.athoracsur.2006.10.089.
21. David TE, Maganti M, Armstrong S. Aortic root aneurysm: principles of repair and long-term follow-up. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S14-9; discussion S45-51. doi:10.1016/j.jtcvs.2010.07.041.
22. Oka T, Okita Y, Matsumori M, Okada K, Minami H, Munakata H, et al. Aortic regurgitation after valve-sparing aortic root replacement: modes of failure. Ann Thorac Surg. 2011;92(5):1639-44. doi:10.1016/j.athoracsur.2011.06.080.
23. David TE, Armstrong S, Manlhiot C, McCrindle BW, Feindel CM. Long-term results of aortic root repair using the reimplantation technique. J Thorac Cardiovasc Surg. 2013;145(3 Suppl):S22-5. doi:10.1016/j.jtcvs.2012.11.075.
24. Coselli JS, Hughes MS, Green SY, Price MD, Zarda S, de la Cruz KI, et al. Valve-sparing aortic root replacement: early and midterm outcomes in 83 patients. Ann Thorac Surg. 2014;97(4):1267-73; discussion 1273-4. doi:10.1016/j.athoracsur.2013.10.076.
25. Kunihara T, Aicher D, Rodionycheva S, Groesdonk HV, Langer F, Sata F, et al. Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair. J Thorac Cardiovasc Surg. 2012;143(6):1389-95. doi:10.1016/j.jtcvs.2011.07.036.
26. Leontyev S, Trommer C, Subramanian S, Lehmann S, Dmitrieva Y, Misfeld M, et al. The outcome after aortic valve-sparing (David) operation in 179 patients: a single-centre experience. Eur J Cardiothorac Surg. 2012;42(2):261-6; discussion 266-7. doi:10.1093/ejcts/ezs011.
27. David TE. Aortic valve sparing operations: outcomes at 20 years. Ann Cardiothorac Surg. 2013;2(1):24-9. doi:10.3978/j.issn.2225-319X.2012.11.15.
28. Richardt D, Stierle U, Sievers HH. Long-term results after aortic valve-sparing-reimplantation operation (David) in bicuspid aortic valve. J Heart Valve Dis. 2015;24(1):4-9. [MedLine]
29. Shrestha ML, Beckmann E, Abd Alhadi F, Krueger H, Meyer-Bockenkamp F, Bertele S, et al. Elective David I procedure has excellent long-term results: 20-year single-center experience. Ann Thorac Surg. 2018;105(3):731-8. doi:10.1016/j.athoracsur.2017.08.040. [MedLine]
30. Schäfers HJ, Aicher D, Langer F, Lausberg HF. Preservation of the bicuspid aortic valve. Ann Thorac Surg. 2007;83(2):S740-5; discussion S785-90. doi:10.1016/j.athoracsur.2006.11.017.
31. Aicher D, Kunihara T, Abou Issa O, Brittner B, Gräber S, Schäfers HJ. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation. 2011;123(2):178-85. doi:10.1161/CIRCULATIONAHA.109.934679.
32. Martens A, Beckmann E, Kaufeld T, Fleissner F, Neuser J, Korte W, et al. Valve-sparing aortic root replacement (David I procedure) in Marfan disease: single-centre 20-year experience in more than 100 patients†. Eur J Cardiothorac Surg. 2019;55(3):476-83. doi:10.1093/ejcts/ezy300. [MedLine]
33. Tian D, Rahnavardi M, Yan TD. Aortic valve sparing operations in aortic root aneurysms: remodeling or reimplantation? Ann Cardiothorac Surg. 2013;2(1):44-52. doi:10.3978/j.issn.2225-319X.2013.01.14. [MedLine]
34. Kremer J, Farag M, Zaradzki M, Szabó G, Ruhparwar A, Kallenbach K, et al. The reimplantation valve-sparing aortic root replacement technique for patients with Marfan syndrome: a single-center experience. Sci Rep. 2019;9(1):12021. doi:10.1038/s41598-019-48572-9.
35. Monsefi N, Zierer A, Risteski P, Primbs P, Miskovic A, Karimian-Tabrizi A, et al. Long-term results of aortic valve resuspension in patients with aortic valve insufficiency and aortic root aneurysm. Interact Cardiovasc Thorac Surg. 2014;18(4):432-7. doi:10.1093/icvts/ivt530.
No financial support.
No conflict of interest.
Authors' roles & responsibilities
GY Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; final approval of the version to be published
AÖ Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; final approval of the version to be published
FÇ Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; final approval of the version to be published
EUÜ Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; final approval of the version to be published
HZI Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; final approval of the version to be published
CLB Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; final approval of the version to be published
AS Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved; final approval of the version to be published
Article receive on Tuesday, March 24, 2020
Article accepted on Tuesday, March 24, 2020
 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license