Dimitrios StarvridisI; Arian Arjomandi RadII; Paola Keese MontanhesiIII; Hristo KirovIV; Max WackerI; Panagiotis TasoudisV; Murat MukharyamovIV; Ricardo E. TremlVI; Jens WippermannI; Torsten DoenstIV; Ibrahim SultanVII; Michel Pompeu SáVII; Tulio CaldonazoIV
DOI: 10.21470/1678-9741-2024-0211
ABSTRACT
Introduction: Minimally invasive techniques for aortic valve replacement have become increasingly popular. The most common minimally invasive approaches are mini-sternotomy and right anterior mini-thoracotomy. We aimed to review the literature and compare clinical outcomes for these two approaches.AVR = Aortic valve replacement
BMI = Body mass index
CCTR = Cochrane Central Register of Controlled Trials
CI = Confidence interval
CKD = Chronic kidney disease
COPD = Chronic obstructive pulmonary disease
CPB = Cardiopulmonary bypass
EuroSCORE = European System for Cardiac Operative Risk Evaluation
ICU = Intensive care unit
LILACS = Literatura Latino-Americana e do Caribe em Ciências da Saúde
LOS = Length of stay
LVEF = Left ventricular ejection fraction
M = Multicenter
MS = Mini-sternotomy
NM = Not multicenter
NP = Not prospective
NR = Not randomized
OR = Odds ratio
PAD = Peripheral artery disease
PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses
RAMT = Right anterior mini-thoracotomy
SD = Standard deviation
SMD = Standard mean difference
INTRODUCTION
Aortic valve replacement (AVR) have expanded dramatically over the years owing to advancements in treatment modalities and technologies[1]. Surgical options encompass conventional and minimally invasive approaches, both showing similar mortality rates[2]. Nevertheless, minimally invasive AVR has become increasingly popular due to its ability to avoid complete sternotomy. It offers several benefits, including reduced ventilation time, shorter intensive care unit (ICU) and hospital stays, lower blood loss and transfusion requirements, reduced atrial fibrillation rates, faster recovery, and better cosmesis[3]. Moreover, it is equally safe and efficient with reduced hospital costs[4].
Mini-sternotomy (MS) and right anterior mini-thoracotomy (RAMT) are the most common minimally invasive approaches to AVR. These surgical techniques offer distinct advantages and technical challenges ranging from surgical exposure to postoperative recovery and outcomes. MS is performed through a 3- to 7-cm midline skin incision with upper partial sternotomy, and it is associated with less postoperative pain, less blood loss, and lower rates of wound infection and dehiscence[2,3]. Conversely, RAMT is performed through a 5- to 7-cm incision in the right second intercostal space without traumatizing the sternum. Comparative data showed lower postoperative pain and shorter ICU length of stay (LOS) with the use of thoracotomy[5,6].
As one of the most performed procedures worldwide, AVR undergoes continuous refinement to improve surgical outcomes, minimize invasiveness, and optimize recovery. However, data comparing MS and RAMT are limited, and there are no randomized trials in the literature.
Salmasi et al.[7] aggregated previously data on this topic showing significant differences regarding postoperative outcomes. Although its central aim was to compare directly these two techniques, this work has some intrinsic limitations that prevent it from providing a clear overview of this subject. For instance, a sensitivity analysis to identify outliers was not performed, and just < 10% of patients (n=2,926) from the current literature were included.
In this context, previous studies with populations basically from single-center registries have demonstrated the safety of RAMT in relation to perioperative mortality[8], however a meta-analytical analysis involving all the major multinational registries on the subject based in the new standards of systematic reviews has not yet been performed. Ultimately, significant innovations marked the last 10 years of minimally invasive cardiac surgery and specially valve therapies. As the current guidelines do not mention any kind of procedure preference in different situations, choosing between the two approaches remains a surgeon’s decision and requires careful consideration.
In this context, we performed a systematic review of the topic and a meta-analysis of contemporary studies to compare major clinical outcomes between the two strategies.
METHODS
Ethical approval of this analysis was not required as no human or animal subjects were involved. This review was registered with the National Institute for Health Research International Registry of Systematic Reviews (CRD42023451208).
Search Strategy
We performed a comprehensive literature search to identify contemporary studies reporting shortand long-term outcomes between patients who underwent AVR with the two different techniques (MS or RAMT). Searches were run on March, 2023, in the following databases: Ovid MEDLINE®, Embase, and Google Scholar. The search strategy is available in Supplementary Table 1.
| Ovid MEDLINE®. |
|---|
| ("aortic valve"[MeSH Terms] OR ("aortic"[All Fields] AND "valve"[All Fields]) OR "aortic valve"[All Fields]) AND ("replace"[All Fields] OR "replaceable"[All Fields] OR "replaced"[All Fields] OR "replaces"[All Fields] OR "replacing"[All Fields] OR "replacment"[All Fields] OR "replantation"[MeSH Terms] OR "replantation"[All Fields] OR "replacement"[All Fields] OR "replacements"[All Fields]) AND ("mini"[All Fields] AND ("sternotomy"[MeSH Terms] OR "sternotomy"[All Fields] OR "sternotomies"[All Fields])) AND ("mini"[All Fields] AND ("thoracotomy"[MeSH Terms] OR "thoracotomy"[All Fields] OR "thoracotomies"[All Fields])) |
| Translations |
| aortic valve: "aortic valve"[MeSH Terms] OR ("aortic"[All Fields] AND "valve"[All
Fields]) OR "aortic valve"[All Fields] replacement: "replace"[All Fields] OR "replaceable"[All Fields] OR "replaced"[All Fields] OR "replaces"[All Fields] OR "replacing"[All Fields] OR "replacment"[All Fields] OR "replantation"[MeSH Terms] OR "replantation"[All Fields] OR "replacement"[All Fields] OR "replacements"[All Fields] sternotomy: "sternotomy"[MeSH Terms] OR "sternotomy"[All Fields] OR "sternotomies"[All Fields] thoracotomy: "thoracotomy"[MeSH Terms] OR "thoracotomy"[All Fields] OR "thoracotomies"[All Fields] Keywords used in Embase and Google Scholar (aortic valve replacement) AND (sternotomy) AND (thoracotomy) |
Study Selection and Data Extraction
The study selection followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) strategy. After de-duplication (i.e., exclusion of records with the same Digital Object Identifier, but with possibly minimal differences in title), the records were screened by two independent reviewers (DS and AAR). Any discrepancies and disagreements were resolved by a third author (TC). Titles and abstracts were reviewed against predefined inclusion and exclusion criteria. Studies were considered for inclusion if they were written in English and reported direct between patients who underwent AVR with the two different techniques (MS or RAMT). Animal studies, abstracts, case reports, commentaries, editorials, expert opinions, conference presentations, and studies which did not report the outcomes of interest were excluded. The full text was pulled for the selected studies for a second round of eligibility screening. References for articles selected were also reviewed for relevant studies not captured by the original search. Studies by the same author comprising the same population were critically analyzed to avoid population overlapping.
The Risk of Bias in Non-Randomized Studies of Interventions (or ROBINS-I) tool was systematically used to assess included studies for risk of bias[9]. The studies and their characteristics were classified into low, moderate, and serious risk of bias. Two independent reviewers (DS and AAR) assessed the risk of bias. When there was a disagreement, a third reviewer (TC) checked the data and made the final decision (Supplementary Figure 1).
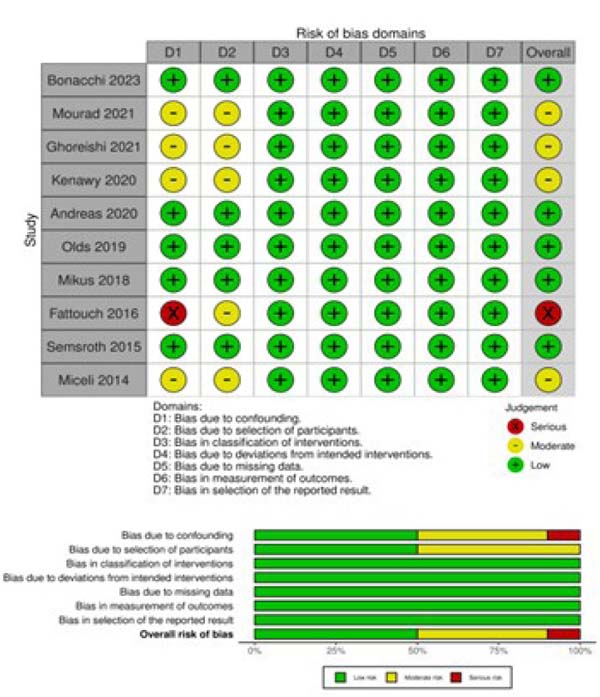
Two reviewers (DS nd AAR) independently performed data extraction. Accuracy was verified by a third author (TC). The extracted variables included study characteristics (publication year, sample size, study design, country, study period, and presence or absence from population adjustment) as well as patient demographics (age, sex, body mass index [BMI], mean left ventricular ejection fraction [LVEF], European System for Cardiac Operative Risk Evaluation [EuroSCORE], hypertension, diabetes, chronic kidney disease, peripheral artery disease [PAD], chronic obstructive pulmonary disease [COPD], and nature of the aortic valve disease nature).
Selected Endpoints
The primary endpoint was perioperative mortality, defined as 30-day or in-hospital mortality. The secondary endpoints were reoperation for bleeding, stroke, operation duration, ICU LOS, cardiopulmonary bypass (CPB) time, cross-clamping time, hospital LOS, paravalvular leak, renal complications, conversion to full sternotomy, permanent pacemaker implantation, and wound infection. Random effects models were performed.
Statistical Analysis
Odds ratio (OR) with 95% confidence interval (CI) and P-values were calculated for each of the clinical outcomes. Standard mean difference (SMD) was calculated for the continuous variables. An OR > 1 indicated that the outcome was more frequently present in the MS group. A SMD > 0 corresponded to longer stay/time in the MS group. Forest plots were created to represent the clinical outcomes. Chi-squared and I2 tests were executed for the assessment of statistical heterogeneity[10]. By using a random effects model, the ORs were combined across the studies[11]. Inherent clinical heterogeneity between the studies was balanced via the implementation of a random effects model[12,13]. Funnel plots were constructed to assess publication bias. All analyses were completed through the “metafor” package of R Statistical Software (version 4.0.2), Foundation for Statistical Computing (Vienna, Austria).
RESULTS
Study Characteristics
A total of 1,123 studies were retrieved from the systematic search, of which 10 met the criteria for inclusion in the final analysis [8,14-22]. Figure 1 shows the PRISMA flowchart for study selection.
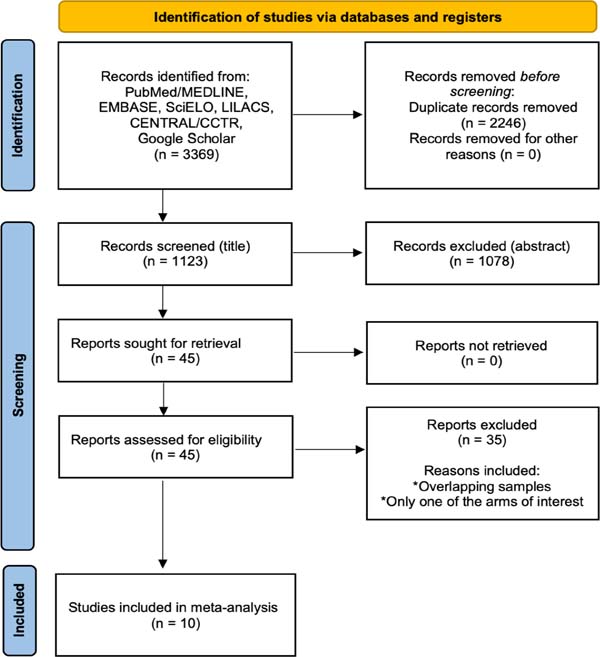
Table 1 shows the details of the included studies. The overall patient number was 30,524, while MS patients were 20,932 and RAMT patients were 9,592. Included studies were published between 2014 and 2023, all the studies were non-randomized, observational, and with retrospective nature. Six studies included risk adjusted populations. Two studies were multinational databases.
| Study | Patients (N) MS/RAMT |
Study design | Country | Study period | Population comparability |
|---|---|---|---|---|---|
| Bonnachi et al. 2023[15] | 1972 986/986 |
NR, NP, M | Italy | 1999-2019 | Propensity score matching |
| Mourad et al. 2021[21] | 260 132/128 |
NR, NP, NM | Egypt | 2014-2020 | No adjustment |
| Ghoreishi et al. 2021[17] | 23925 17306/6619 |
NR, NP, M | United States of America | 2011-2017 | Multivariable logistic regression |
| Kenawy et al. 2020 [18] | 232 126/106 |
NR, NP, NM | United Kingdom | 2015-2019 | No adjustment |
| Andreas et al. 2020 [14] | 1138 569/569 |
NR, NP, M | Multinational register | 2013-2020 | Propensity score matching |
| Olds et al. 2019 [8] | 387 267/120 |
NR, NP, NM | United States of America | 2012-2015 | Multivariable logistic regression |
| Mikus et al. 2018 [20] | 754 377/377 |
NR, NP, NM | Italy | 2010-2017 | Propensity score matching |
| Fattouch et al. 2016 [16] | 1130 854/276 |
NR, NP, M | Italy | 2010-2014 | No adjustment |
| Semsroth et al. 2015 [22] | 320 160/160 |
NR, NP, NM | Austria | 2001-2012 | Propensity score matching |
| Miceli et al. 2014 [19] | 406 155/251 |
NR, NP, NM | Italy | 2005-2011 | No adjustment |
M=multicenter; MS=mini-sternotomy; NM=not multicenter; NP=not prospective; NR=not randomized; RAMT=right anterior mini-thoracotomy
Patient Characteristics
Supplementary Table 2 summarizes the demographic data of the overall patient population. There was no relevant difference regarding age, sex, BMI, LVEF, EuroSCORE, presence of hypertension, diabetes, PAD, COPD, and combined aortic disease.
| Mini-sternotomy | Right Anterior Mini-thoracotomy | |
|---|---|---|
| Age (years) Mean ± SD (total) |
69.47 ± 11.72 (20785) |
70.62 ± 11.46 (9739) |
| Female sex (%) (N/Total) |
42.1% (8767/20785) |
43.1% (4198/9739) |
| BMI (kg/m2) Mean ± SD (Total) |
29.12 ± 5.89 (20510) |
28.57 ± 5.87 (9221) |
| LVEF (%) Mean ± SD (Total) |
59.12 + 9.81 (19645) |
57.98 + 9.82 (9197) |
| EuroSCORE Mean ± SD (Total) |
2.81 ± 3.03 (20367) |
3.29 ± 3.0 (9345) |
| Hypertension (%) (N/Total) |
74.4% (15484/20625) |
73.8% (7074 / 9579) |
| Type II diabetes % (N/Total) |
26.8% (5571/20785) |
26.5% (2581/9739) |
| CKD % (N/Total) |
5.5% (1078/19484) |
10.5% (941/8924) |
| PAD % (N/Total) |
10.1% (1916/18824) |
13.4% (1111/8233) |
| COPD (%) (N/Total) |
20.7% (4168/20092) |
21.9% (1939/8827) |
| Aortic stenosis % (N/Total) |
86.9% (17970/20659) |
81.3% (7840/9633) |
| Aortic regurgitation % (N/Total) |
64.6% (13346/20659) |
57.3% (5520/9633) |
| Combined aortic disease % (N/Total) |
24.7% (762/3073) |
29.4% (762/2587) |
BMI=body mass index; CKD=chronic kidney disease; COPD=chronic obstructive pulmonary disease; EuroSCORE=European System for Cardiac Operative Risk Evaluation; LVEF=left ventricular ejection fraction; PAD=peripheral artery disease; SD=standard deviation.
Meta-Analysis
Central Image and Table 2 outline the detailed results of the meta-analysis.
| Outcome | Number of studies | Number of patients | Effect estimate (95% CI; P-value) |
|---|---|---|---|
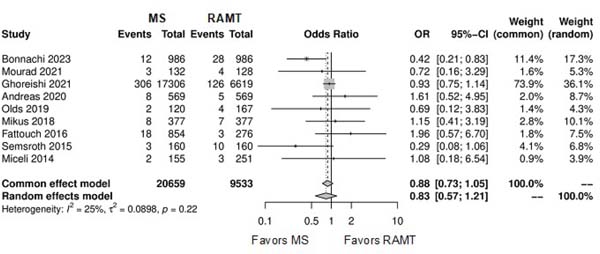
| Perioperative mortality | 9 | 30,192 | OR: 0.83; 95% CI 0.57-1.21; P=0.33 |
| Reoperation for bleeding | 9 | 29,396 | OR: 0.69; 95% CI 0.50-0.97; P=0.03 |
| Stroke | 10 | 30,524 | OR: 1.27; 95% CI 1.01-1.60; P=0.04 |
| Operation duration | 3 | 26,661 | SMD: -0.58; 95% CI -1.01 to -0.14; P=0.01 |
| Intensive care unit length of stay | 9 | 30,118 | SMD: 0.01; 95% CI -0.41 to 0.43; P=0.95 |
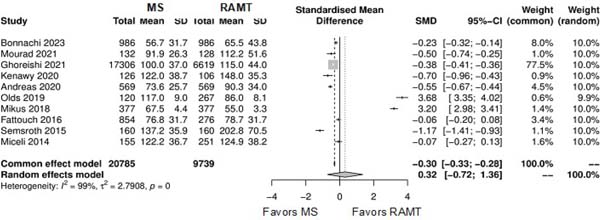
| Cardiopulmonary bypass time | 10 | 30,524 | SMD: 0.32; 95% CI -0.72 to 1.36; P=0.54 |
| Cross-clamping time | 10 | 30,524 | SMD: 0.32; 95% CI -0.72 to 1.36; P=0.53 |
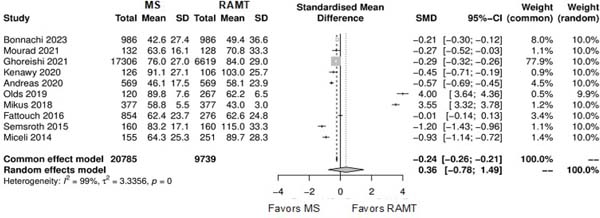
| Hospital length of stay | 8 | 29,45 | SMD: 0.00; 95% CI -0.34 to 0.34; P=0.11 |
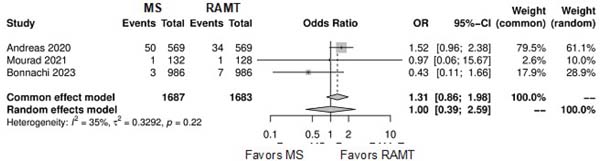
| Paravalvular leak | 3 | 3,37 | OR: 1.00; 95% CI 0.39-2.59; P=0.99 |
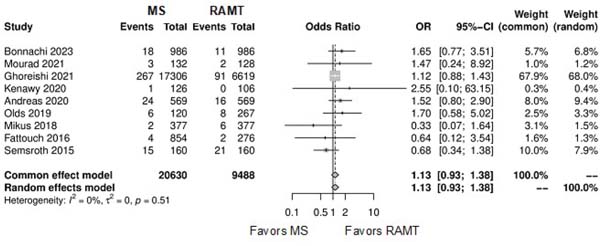
| Renal complications | 9 | 30,118 | OR: 1.13; 95% CI 0.93-1.38; P=0.20 |
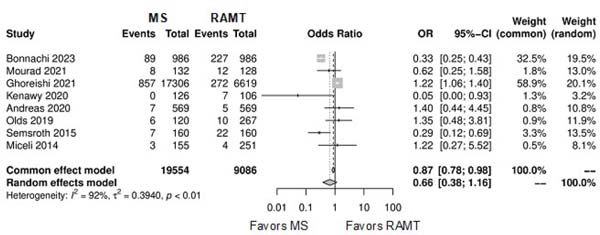
| Conversion to full sternotomy | 8 | 28,64 | OR: 0.66; 95% CI 0.38-1.16; P=0.15 |
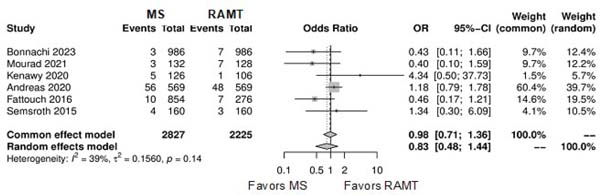
| Permanent pacemaker implantation | 6 | 5,052 | OR: 0.83; 95% CI 0.48-1.44; P=0.50 |
| Wound infection | 8 | 29,731 | OR: 1.05; 95% CI 0.54-2.05; P=0.88 |
CI=confidence interval; OR=odds ratio; SMD=standard mean difference
Primary Endpoint
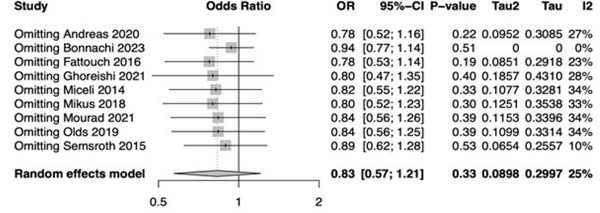
Figure 2 shows the forest plot for perioperative mortality. There was not a statistical significance between the two approaches (OR: 0.83; 95% CI 0.57-1.21; P=0.33). Supplementary Figure 2 shows the leave-one-out analysis showing that most of the studies confirm the robustness of the analysis, with minimal variations of the CI. Supplementary Figure 3 provides the funnel plot for the publication bias assessment.
Secondary Outcomes
Figure 3 shows the forest plot for reoperation for bleeding. The RAMT group showed significantly higher rates of reoperation for bleeding in comparison with the MS group (OR: 0.69; 95% CI 0.50-0.97; P=0.03).
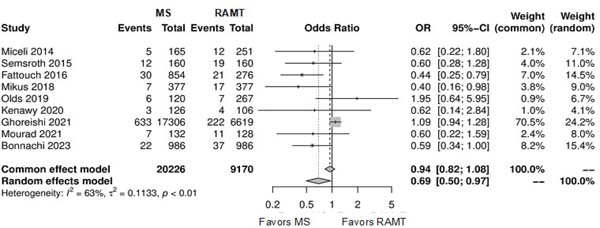
Figure 4 shows the forest plot for stroke. The RAMT group showed significantly lower rates of stroke in comparison with the MS group (OR: 1.27; 95% CI 1.01-1.60; P=0.04).
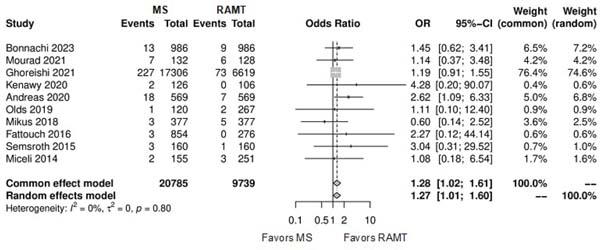
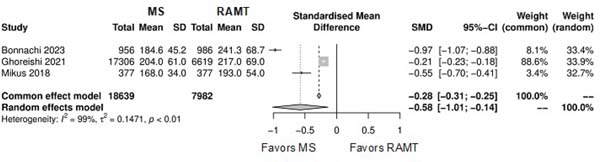
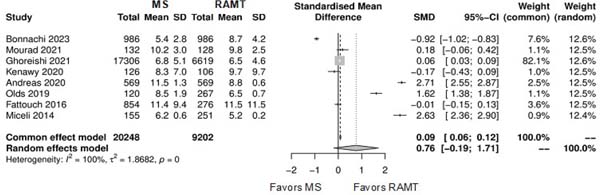
Figure 5 shows the forest plot for operation duration. The RAMT group showed significantly longer operation duration in comparison with the MS group (SMD: -0.58; 95% CI -1.01 to -0.14; P=0.01).
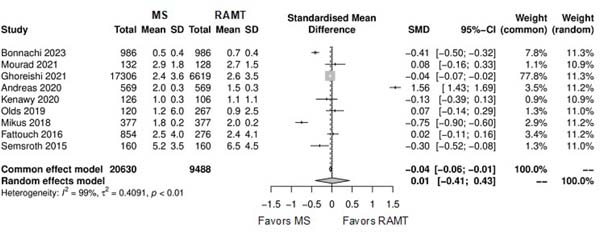
Further comparison regarding ICU LOS, CPB time, cross-clamping time, hospital LOS, paravalvular leak, renal complications, conversion to full sternotomy, permanent pacemaker implantation, and wound infection were not statistically significant (Supplementary Figures 4-12). Information regarding the valve types “sutureless vs. stented” or “tissue vs. mechanic” were only mentioned in two of the studies[14,15].
DISCUSSION
The analysis suggests that both techniques present similar perioperative mortality rates for AVR. However, RAMT is associated with higher rates of reoperation for bleeding, lower rates of stroke and longer operation duration time. There was no difference regarding ICU LOS, CPB time, cross-clamping time, hospital LOS, paravalvular leak, renal complications, conversion to full sternotomy, permanent pacemaker implantation, and wound infection.
Minimally invasive alternatives for AVR have demonstrated comparable safety and efficacy to conventional median sternotomy[6,8]. Their ability to provide favorable clinical outcomes is associated with advancements in surgical techniques, instrumentation, and perioperative care[6,8]. This paradigm shift towards less invasive approaches has been driven by the pursuit of reduced morbidity, shorter hospital stays, expedited recovery, and greater patient satisfaction. Although this meta-analysis did not show any advantage in relation to any of the minimally invasive techniques when compared to each other, there is a relative reduction in postoperative pain associated with faster mobilization and recovery when comparing these techniques to traditional AVR with complete sternotomy[23-25].
Previous comparisons between MS and RAMT provided valuable insights into their respective benefits and limitations[8,14-22], but the best approach remains on debate. This systematic review and meta-analysis demonstrated equivalent perioperative mortality rates between the two groups, what emphasizes the competency of surgeons in performing both techniques and the overall safety of minimally invasive AVR.
Additionally, RAMT showed a higher rate of reoperation for bleeding when compared to MS. This finding suggests that MS may offer more effective hemostasis strategies due to better visualization, resulting in fewer postoperative bleeding complications. However, it does not decrease the safety of RAMT, as the bleeding events were not related to higher perioperative mortality in this analysis. The information is, however, relevant for special patient populations, for instance, Jehovah’s witnesses.
An important and curious fact about the work is the fact that MS was associated with more frequent stroke events. Although both techniques demand aortic cross-clamping for the surgery, the majority of RAMT procedures are performed using femoral cannulation, and it could be unexpectedly related with the reduction of cerebrovascular events[26,27].
Furthermore, the longer operation times observed with RAMT may be attributed to the learning curve associated with this technique. As surgeons become more experienced with RAMT, operation times could potentially decrease, enhancing overall surgical efficiency[28,29].
The choice between MS and RAMT is influenced by various factors, including surgeon experience and patient-specific considerations. The analysis revealed that most of the included studies had larger cohorts of MS patients, possibly indicating a preference for this technique among surgeons due to familiarity or perceived ease of execution. This raises questions about the impact of surgeon experience on the outcomes and preferences for a specific technique.
It's worth acknowledging that the lack of standardized reporting regarding valve types and replacement methods introduces heterogeneity into the analysis. The type of valve used and the method of replacement can also influence operation duration and, consequently, patient outcomes. As newer technology and instruments continue to develop, the creation of modern rapid deployment stented valves and refined instrumentation can contribute to enhanced patient safety and long-term outcomes for minimally invasive AVR. Finally, as transcatheter aortic valve replacement gains prominence, there is an increasing demand for surgical solutions that offer superior cosmetic results, shorter hospital stays, and long-term durability. The insights gained from this analysis contribute to the ongoing dialogue surrounding the selection of surgical approaches, particularly in the context of evolving patient expectations and advancing technology.
Study Strength and Limitations
We analyzed 12 different outcomes besides mortality. Six out of ten studies presented data from risk-adjusted populations and most of them had a well-designed methodological approach. However, this work has the intrinsic limitations of observational series, including the risk of methodological heterogeneity of the included studies and residual confounders. In addition, treatment allocation bias is likely present in all observational series comparing two therapies with different invasiveness. Ghoreishi et al. provided a significant number of patients compared to the other studies[17]. Finally, information regarding whether the same surgeons performed both techniques or not was not mentioned in all of the studies. Besides that, there was not enough data in the studies regarding the type of prosthesis used, which can substantially influence long-term results, and regarding the complications resulting from peripheral cannulation. Precisely for this reason, this work has an important role in generating hypotheses and indicating possible clinical correlations between clinical events that can definitely support the design of new RCTs. Therefore, this meta-analysis based on observational studies has per se the limitation of not being able to produce relationships with a causal character.
CONCLUSION
The analysis suggests that both techniques present similar perioperative mortality rates for AVR. However, RAMT is associated with higher rates of reoperation for bleeding, lower rates of stroke, and longer operation duration time. There was no difference regarding ICU LOS, CPB time, cross-clamping time, hospital LOS, paravalvular leak, renal complications, conversion to full-sternotomy, permanent pacemaker implantation, and wound infection.
REFERENCES
1. Spadaccio C, Alkhamees K, Al-Attar N. Recent advances in aortic valve replacement. F1000Res. 2019;8:F1000 Faculty Rev-1159. doi:10.12688/ f1000research.17995.1.
2. Oo S, Khan A, Chan J, Juneja S, Caputo M, Angelini G, et al. Propensity matched analysis of minimally invasive versus conventional isolated aortic valve replacement. Perfusion. 2023;38(2):261-9. doi:10.1177/02676591211045802.
3. Chang C, Raza S, Altarabsheh SE, Delozier S, Sharma UM, Zia A, et al. Minimally invasive approaches to surgical aortic valve replacement: a meta-analysis. Ann Thorac Surg. 2018;106(6):1881-9. doi:10.1016/j.athoracsur.2018.07.018.
4. Hassan M, Miao Y, Maraey A, Lincoln J, Brown S, Windsor J, et al. Minimally invasive aortic valve replacement: cost-benefit analysis of ministernotomy versus minithoracotomy approach. J Heart Valve Dis. 2015;24(5):531-9.
5. Gilmanov D, Farneti PA, Ferrarini M, Santarelli F, Murzi M, Miceli A, et al. Full sternotomy versus right anterior minithoracotomy for isolated aortic valve replacement in octogenarians: a propensity-matched study †. Interact Cardiovasc Thorac Surg. 2015;20(6):732-41; discussion 741. doi:10.1093/icvts/ivv030.
6. Glauber M, Gilmanov D, Farneti PA, Kallushi E, Miceli A, Chiaramonti F, et al. Right anterior minithoracotomy for aortic valve replacement: 10-year experience of a single center. J Thorac Cardiovasc Surg. 2015;150(3):548-56.e2. doi:10.1016/j.jtcvs.2015.06.045.
7. Yousuf Salmasi M, Hamilton H, Rahman I, Chien L, Rival P, Benedetto U, et al. Mini-sternotomy vs right anterior thoracotomy for aortic valve replacement. J Card Surg. 2020;35(7):1570-82. doi:10.1111/jocs.14607.
8. Olds A, Saadat S, Azzolini A, Dombrovskiy V, Odroniec K, Lemaire A, et al. Improved operative and recovery times with mini-thoracotomy aortic valve replacement. J Cardiothorac Surg. 2019;14(1):91. doi:10.1186/s13019-019-0912-0.
9. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919.
10. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-60. doi:10.1136/bmj.327.7414.557.
11. Jackson D, Law M, Stijnen T, Viechtbauer W, White IR. A comparison of seven random-effects models for meta-analyses that estimate the summary odds ratio. Stat Med. 2018;37(7):1059- 85. doi:10.1002/sim.7588.
12. Verhagen AP, Ferreira ML. Forest plots. J Physiother. 2014;60(3):170- 3. doi:10.1016/j.jphys.2014.06.021.
13. Chu H, Chen S, Louis TA. Random effects models in a meta-analysis of the accuracy of two diagnostic tests without a gold standard. J Am Stat Assoc. 2009;104(486):512-23. doi:10.1198/jasa.2009.0017.
14. Andreas M, Berretta P, Solinas M, Santarpino G, Kappert U, Fiore A, et al. Minimally invasive access type related to outcomes of sutureless and rapid deployment valves. Eur J Cardiothorac Surg. 2020;58(5):1063-71. doi:10.1093/ejcts/ezaa154.
15. Bonacchi M, Dokollari A, Parise O, Sani G, Prifti E, Bisleri G, et al. Ministernotomy compared with right anterior minithoracotomy for aortic valve surgery. J Thorac Cardiovasc Surg. 2023;165(3):1022- 32.e2. doi:10.1016/j.jtcvs.2021.03.125.
16. Fattouch K, Moscarelli M, Del Giglio M, Albertini A, Comoglio C, Coppola R, et al. Non-sutureless minimally invasive aortic valve replacement: mini-sternotomy versus mini-thoracotomy: a series of 1130 patients. Interact Cardiovasc Thorac Surg. 2016;23(2):253- 8. doi:10.1093/icvts/ivw104.
17. Ghoreishi M, Thourani VH, Badhwar V, Massad M, Svensson L, Taylor BS, et al. Less-invasive aortic valve replacement: trends and outcomes from the society of thoracic surgeons database. Ann Thorac Surg. 2021;111(4):1216-23. doi:10.1016/j.athoracsur.2020.06.039.
18. Kenawy A, Abdelbar A, Tennyson C, Taylor R, Zacharias J. Is it safe to move away from a full sternotomy for aortic valve replacement? Asian Cardiovasc Thorac Ann. 2020;28(9):553-9. doi:10.1177/0218492320948321.
19. Miceli A, Murzi M, Gilmanov D, Fugà R, Ferrarini M, Solinas M, et al. Minimally invasive aortic valve replacement using right minithoracotomy is associated with better outcomes than ministernotomy. J Thorac Cardiovasc Surg. 2014;148(1):133-7. doi:10.1016/j.jtcvs.2013.07.060.
20. Mikus E, Calvi S, Campo G, Pavasini R, Paris M, Raviola E, et al. Full sternotomy, hemisternotomy, and minithoracotomy for aortic valve surgery: is there a difference? Ann Thorac Surg. 2018;106(6):1782- 8. doi:10.1016/j.athoracsur.2018.07.019.
21. Mourad F, Abd Al Jawad M. Mini sternotomy and mini thoracotomy for aortic valve replacement: is there a difference? Heart Surg Forum. 2021;24(5):E855-9. doi:10.1532/hsf.4029.
22. Semsroth S, Matteucci-Gothe R, Heinz A, Dal Capello T, Kilo J, Müller L, et al. Comparison of anterolateral minithoracotomy versus partial upper hemisternotomy in aortic valve replacement. Ann Thorac Surg. 2015;100(3):868-73. doi:10.1016/j.athoracsur.2015.03.009.
23. Klop IDG, Van Putte BP, Kloppenburg GTL, Klautz RJM, Sprangers MAG, Nieuwkerk PT, et al. Postoperative quality of life and pain after upper hemisternotomy and conventional median sternotomy for aortic valve replacement: results of a randomized clinical trial. Interdiscip Cardiovasc Thorac Surg. 2024;38(5):ivae083. doi:10.1093/icvts/ivae083.
24. Walther T, Falk V, Metz S, Diegeler A, Battellini R, Autschbach R, et al. Pain and quality of life after minimally invasive versus conventional cardiac surgery. Ann Thorac Surg. 1999;67(6):1643-7. doi:10.1016/ s0003-4975(99)00284-2.
25. Gebauer A, Konertz J, Petersen J, Brickwedel J, Köster D, Schulte- Uentrop L, et al. The impact of a standardized enhanced recovery after surgery (ERAS) protocol in patients undergoing minimally invasive heart valve surgery. PLoS One. 2023;18(3):e0283652. doi:10.1371/journal.pone.0283652.
26. Ramchandani M, Al Jabbari O, Abu Saleh WK, Ramlawi B. Cannulation strategies and pitfalls in minimally invasive cardiac surgery. Methodist Debakey Cardiovasc J. 2016;12(1):10-3. doi:10.14797/mdcj-12-1-10.
27. Saadat S, Schultheis M, Azzolini A, Romero J, Dombrovskiy V, Odroniec K, et al. Femoral cannulation: a safe vascular access option for cardiopulmonary bypass in minimally invasive cardiac surgery. Perfusion. 2016;31(2):131-4. doi:10.1177/0267659115588631.
28. Liang NE, Wisneski AD, Wozniak CJ, Ge L, Tseng EE. Evolution of minimally invasive surgical aortic valve replacement at a veterans affairs medical center. Innovations (Phila). 2019;14(3):251-62. doi:10.1177/1556984519843498.
29. Masuda T, Nakamura Y, Ito Y, Kuroda M, Nishijima S, Okuzono Y, et al. The learning curve of minimally invasive aortic valve replacement for aortic valve stenosis. Gen Thorac Cardiovasc Surg. 2020;68(6):565-70. doi:10.1007/s11748-019-01234-z.
Authors’Roles & Responsibilities
DS = Substantial contributions to the design of the work; and the acquisition and analysis of data for the work; drafting the work; final approval of the version to be published
AAR = Substantial contributions to the design of the work; and the acquisition and analysis of data for the work; drafting the work; final approval of the version to be published
PKM = Substantial contributions to the analysis and interpretation of data for the work; final approval of the version to be published
HK = Substantial contributions to the analysis and interpretation of data for the work; drafting the work; final approval of the version to be published
MW = Substantial contributions to the analysis and interpretation of data for the work; drafting the work; final approval of the version to be published
PT = Substantial contributions to the analysis and interpretation of data for the work; drafting the work; final approval of the version to be published
MM = Substantial contributions to the analysis and interpretation of data for the work; drafting the work; final approval of the version to be published
RET Substantial contributions to the analysis and interpretation of data for the work; drafting the work; final approval of the version to be published
JW = Substantial contributions to the analysis and interpretation of data for the work; drafting the work and revising it; final approval of the version to be published
TD = Substantial contributions to the analysis and interpretation of data for the work; drafting the work and revising it; final approval of the version to be published
IS = Substantial contributions to the analysis and interpretation of data for the work; drafting the work and revising it; final approval of the version to be published
MPS = Substantial contributions to the design of the work; and the analysis and interpretation of data for the work; drafting the work and revising it; final approval of the version to be published
TC = Substantial contributions to the design of the work; and the analysis and interpretation of data for the work; drafting the work and revising it; final approval of the version to be published
Article receive on Monday, June 24, 2024
Article accepted on Friday, September 6, 2024
 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license