OBJECTIVE: To assess the effects of methylene blue infusion on on-pump coronary artery bypass patients in relation to hemodynamic, laboratorial and systemic inflammatory response.
METHOD: Sixty patients were randomized in two groups. Methylene blue was infused in one group. Blood samples were collected before the anesthesia and, 3, 6, 24 and 48 hours after the end of the extracoporeal circulation to determine the IL-1b, IL-6, IL-8, IL-10, IL-12p40, TNFa and nitric oxide levels and perform gasometry and other routine tests.
RESULTS: In the methylene group we observed at different stages, higher systemic vascular resistance, lower TNFa concentrations, fewer leukocytes and neutrophils as well as lower level of nitric oxide. No adverse effects were evidenced.
CONCLUSIONS: Methylene blue infusion showed less tendency of systemic inflammatory responses, lower levels of nitric oxide and a better hemodynamic performance.
OBJETIVO: Estudar em pacientes submetidos à cirurgia de revascularização miocárdica com circulação extracorpórea os efeitos da infusão de azul de metileno na reação inflamatória sistêmica e nas condições hemodinâmica e laboratorial.
MÉTODO: Foram estudados 60 pacientes randomizados em dois grupos, utilizando-se a infusão de azul de metileno em um dos dois grupos. Amostras de sangue foram colhidas antes da indução anestésica, 3, 6, 24 e 48 horas após o término da circulação extracorpórea para dosagens dos marcadores de inflamação (IL-1b, IL-6, IL-8, IL-10, IL-12p40 e TNFa), NO, gasometria e outras dosagens de rotina.
RESULTADOS: O grupo que utilizou azul de metileno mostrou, em diferentes momentos das coletas, maior resistência vascular sistêmica, menor concentração de TNFa, menor número de leucócitos e neutrófilos e menor nível de óxido nítrico. Não ocorreram efeitos adversos importantes.
CONCLUSÕES: A infusão de azul de metileno não evidenciou alterações clínicas ou pulmonares adversas, mostrando uma tendência menor à resposta inflamatória sistêmica, menores níveis de óxido nítrico e melhor estabilidade hemodinâmica.
INTRODUCTION
The use of cardiopulmonary bypass (CPB) in heart surgery induces inflammatory alterations which have been recognized since the 1970s, as published in a report by PARKER et al. [1].
Reduction in the systemic vascular resistance associated with arterial hypertension, denominated by GOMES et al. [2] as vasoplegic syndrome, has a correlation with systemic inflammatory response syndrome (SIRS).
Recent works have shown that the infusion of methylene blue improves the conditions of systemic vascular resistance and arterial pressure, diminishing or even eliminating the necessity of the use of catecolamines [3-6].
Salaris et al. [7] reported the efficiency of methylene blue in the prevention of injury to the tissue of the liver and kidneys due to free radicals in an in vitro model of ischemia / reperfusion.
There is, according to Finkel et al. [8], a modulating correlation between the cytokines and nitric oxide and as methylene blue blocks the production of nitric oxide, according to Mayer et al. [9] there is a possibility of utilizing this substance to prevent SIRS in surgeries using CPB.
OBJECTIVE
This study enrolled patients submitted to coronary artery bypass grafting with CPB, divided in two groups, one of which received methylene blue and the other not. The aim of this work was to study:
1. the systemic inflammatory response by determination of the interleukins and nitric oxide;
2. and the hemodynamic and laboratorial conditions.
METHOD
All the patients gave written consent and the work was approved by the Ethics Committee of the Santa Izabel Hospital of the Santa Casa de Misericórdia of Bahia and the Medical School of the University of São Paulo.
Induction of anesthesia was made with midazolam, sufentanil and pancuronium and maintenance was with sevoflurane. Antibiotic therapy was achieved using cephalotin and methylprednisolone at 30 mg/kg.
After measurement of the hemodynamic parameters, an infusion of methylene blue was initiated in half the patients chosen at random at a dose of 2 mg/kg of weight diluted in a 5% glucose solution over a 6-hour period independently of the surgical time. (Ampoules of 5 mL at 1% - Pharmacy of the Hospital das Clinicas, Medical School, University of São Paulo).
The surgery was performed using CPB and light to moderate hypothermia with anterograde normothermic cardioplegia.
Blood samples were drawn at 3, 6, 24 and 48 hours after the withdrawal of CPB. The blood samples to measure the IL-1b, IL-6, IL-8, IL-10 and TNFa cytokines were sent to the laboratory of Pathology and Molecular biology of the Osvaldo Cruz Foundation, Bahia. Blood samples for the measurement of the nitric oxide were sent to the Laboratory of Inflammation and Pain of the Medical School of Ribeirão Preto.
Other routine measurements were performed in the Santa Izabel Hospital.
Statistical Analysis
The data were stored and analyzed using SPSS 10.0 software. Continuous variables were presented as tendency measurements (arithmetic means) and dispersion measurements (standard deviation - SD). The Student t-test was utilized for the comparison of means and the non-parametric Mann-Whitney test for comparison of means when the variances were not homogenous. For the comparison of proportions, the chi-squared test was used (c2) or the Fisher exact test, the latter when the stratified samples were too small. A level of 5% was considered significant.
RESULTS
1. Characterization of the patients and intra-operative data.
All the patients were evaluated in relation to age, gender, race, smoking, arterial hypertension, diabetes, unstable angina, previous infarctions, CPB time, aortic clamping time and number of grafts performed.
No significant differences were observed.
2. Postoperative complaints
The following variables were evaluated: the existence of blue-tinged urine and/or feces, diarrhea, nausea, cephalea, dizziness, asthenia and dyspnea. Blue-tinged urine continued for up to four postoperative days and was evidenced in 37.9% of the patients of the group who used methylene blue (p-value < 0.05) and in none of the control group. This was the only characteristic with significantly difference between the two groups.
3. Laboratorial variables
Significantly larger values in the leukocyte and neutrophil counts were encountered in the control and experimental groups 48 hours after the initiation of CPB. These values were 16.311 ± 3.974 vs. 13.180 ± 4.355 and 13.782 ± 3.854 vs. 10.883 ± 3.723 respectively. For all the other variables, no significant differences were evidenced (Figures 1 and 2).
4. Gasometric variables pH, Na+ and K+
The determined or calculated variables did not demonstrate real differences between the two groups except in relation to K+ in the 48th hour post-CPB, although this remained within the normal range.
5. Hemodynamic variables
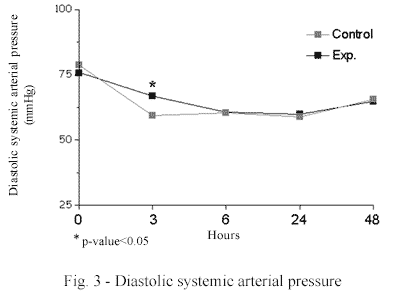
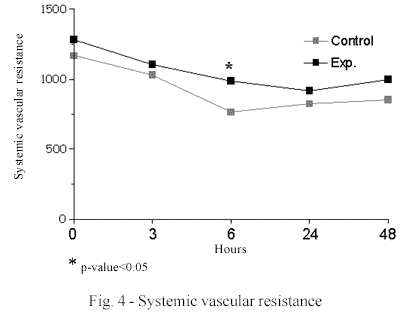
The diastolic systemic arterial pressure (DSAP) was greater in the MB group that than in the control group (67.0 mmHg ± 12.6 vs. 59.5 mmHg ± 12.2; p-value 0.05) in measurements after 3 hours. The systemic vascular resistance (SVR) was greater in the MB group than in the control group 6 hours post-CPB (987.4 dyn.s.cm-5 ± 414.8 vs. 764.4 dyn.s.cm-5 ± 349.1 p-value < 0.05). No significant differences were seen in the other evaluated variables (Figures 3 and 4).
6. Immunomolecular variables and nitric oxide
The measurements of IL-1-b, IL-6, IL-8, IL-10, and IL-12p40 did not give significant differences between the two groups of patients studied (Figures 5, 6, 7 and Table 1).
TNFa gave significantly higher differences in the control group than in the MB group in the 3rd and 48th hours after CPB (Figure 5 and Table 1).
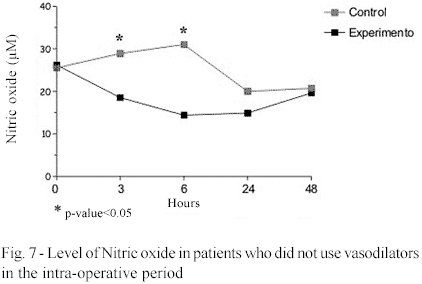
The nitric oxide was significantly higher in the control group in the 6th hour post-CPB. In those patients who did not use vasodilatory drugs in the postoperative period, the nitric oxide was greater in the control group in the 3rd and 6th hours post-CPB giving a significant difference (p-value < 0.05) (Figures 6 and 7 and Table 1).
COMMENTS
The harmful effects of CPB, with alterations in the brain, lungs, heart, kidneys, liver and coagulation and systemic vascular resistance functions, are well known and have a varied etiopathogenesis [1,10-17].
The systemic inflammatory response syndrome is today considered one of the most important factors in the genesis of these alterations [10,14-16,18].
Methylene blue has been reported in the literature as being a drug capable of reverting significant arterial hypotension which does not respond to catecholamines, reducing or even eliminating their necessity. It was initially used in the treatment of arterial hypotension due to septic shock and, more recently, in patients submitted to heart surgery with vasoplegic symptoms [3-6, 19-21].
Nitric oxide is seen in high levels, both during septic shock as in postoperative vasoplegia [20-23], although Brett et al. [24] did not observe an increase in the postoperative period of heart surgery. The increase in the levels of nitric oxide and the cytokines are related to dysfunction of differing systems [11-13].
Methylene blue, by means of several mechanisms such as inhibition of the action of nitric oxide in the vascular smooth muscle and reduction of ischemia and reperfusion, could have beneficial effects in patients submitted to heart surgery with CPB [3-7,9,20].
The systemic inflammatory response triggered by classical and alternative means has ischemia/reperfusion as one of its triggered factors. This leads to an action on the endothelium that regulates the vascular tonus with production of nitric oxide, prostacyclin, thromboxane and endothelin. Methylene blue might, therefore, prevent SIRS or diminish its vascular effects.
The current prospective randomized study was planned to evaluate the effects of methylene blue in patients submitted to CABG surgery with CPB. The preoperative, demographic and intra-operative data were compared and did not present with significant statistical differences, demonstrating the effectiveness of the randomization.
In relation to the adverse effects of the methylene blue, there were no significant differences in the clinical alterations in the two groups, except for blue-tinged urine. Similarly, in the laboratorial examinations, only an increased level of K+ 24-hours after removal of CPB in the MB group was observed but the levels remained within the normal range levels. There were no significant differences between the values of the pulmonary pressures, the pulmonary vascular resistance and gasometric variables. Therefore no significant adverse effects were confirmed. In the literature, the effects of methylene blue on the pulmonary vascular system are controversial. ANDRADE et al. [3] observed among six patients treated with methylene blue, that the SVR increased three, remained unaltered in two and reduced in one. KOELZOW et al. [5] studied two groups of liver transplantation patients one group that used methylene blue and one that did not and confirmed the pulmonary pressures and resistance increased in both groups after the procedure. However, there was no significant difference between the two groups in respect to these parameters. PREISER et al. [21], in patients suffering septic shock that received methylene blue, reported that there was no alteration in the pulmonary pressure nor in the arterial gases, including the supply and demand of oxygen. However, WEINGARTNER et al. [23] verified a slight increase in the rate of pulmonary vascular resistance and a worsening in the pulmonary function, without, however, alterations occurring in the filling of the heart and its outflow.
In the evaluation of SIRS, in this study, it was confirmed that both the pro-inflammatory (IL-1â, IL-6, IL-8 and TNFa) and the anti-inflammatory (IL-10 and IL-12p40) cytokines demonstrated an increase in both groups evidencing the presence of SIRS after heart surgery with CPB. These findings are in agreement with other publications [13,25].
In relation to the comparative study of the cytokines in the two groups, which is one of the objectives of this study, no significant difference was seen between the two groups at any of the time intervals in relation to IL1â, IL-6, IL-8, IL-10 and IL-12p40. The levels of TNFa showed lower values in the MB group in the 3rd and 48th hours in the post-CPB period, demonstrating that the methylene blue reduced production but had no effect on the other cytokines. No references in the literature were found in relation to the influence of methylene blue on SIRS.
There was a significantly lower absolute number of both leukocytes and neutrophils in the methylene blue group at the end of 48 hours post-CPB. Although, in isolation, this finding is not definitive, it suggests a less severe SIRS in the MB group.
In relation to the hemodynamic evaluation, this study confirmed that the systemic vascular resistance was higher in the MB group in all the periods of the experiments and significantly higher in the 6th hour post-CPB. Additionally, the diastolic systemic arterial pressure was significantly higher in the 3rd hour post-CPB in the MB group. This result demonstrates a better arterial vascular tonus as was expected by the mechanism of the action of methylene blue and this was demonstrated in the literature in several clinical conditions such as septic shock and also heart surgery [3-6,21,23].
Determination of the nitric oxide, in this study demonstrated lower values in the MB group from the 3rd to 24th hours post-CPB, with significant differences in the 6th hour. When the group of patients who did not receive vasodilators is evaluated, the difference was significant in the period from 3 to 6 hours. These findings were expected as the effect of methylene blue is known to reduce or to inhibit the synthesis of nitric oxide by the mechanism of inhibiting the nitric oxide synthesis. The proven effect of methylene blue is the inhibition of the guanylyl cyclase, impeding the increase of cGMP or acting as an artificial receptor of electrons, thus inhibiting the formation of free radicals [7,9]. The lower levels of nitric oxide might explain the greater values of systemic vascular resistance that are observed in the hemodynamic evaluation, but the most probable mechanism is a reduction in the formation of cGMP by the inhibition of guanylyl cyclase.
It was verified that only the determinations of nitric oxide demonstrated an inhibitory character of methylene blue, but the markers of SIRS and the hemodynamic parameters did not have a uniform behavior. It should be stressed, however, that the dose of methylene blue utilized was very low and this may have influenced the results.
CONCLUSIONS
In this prospective randomized study of patients submitted to CABG surgery using CPB, we concluded that the use of methylene blue:
1. did not demonstrate adverse clinical effects especially in gas exchange and pulmonary hemodynamics;
2. demonstrated a tendency of lower systemic inflammatory response evaluated by the levels of TNFa and the leukocyte and neutrophil numbers. The levels of the other measured cytokines did not demonstrate significant differences between the patients who received a venous infusion of methylene blue and those who did not;
3. demonstrated a significant action in the inhibition of the production of nitric oxide;
4. demonstrated significantly higher values in the systemic vascular resistance and diastolic systemic arterial pressure in one of the postoperative periods, suggesting a greater hemodynamic stability.
ACKNOWLEDGEMENTS
Eliana Almeida G. Reis, Rita de Cássia Ribeiro Silva, Fernando de Queiroz Cunha and Giuliana Bertozi Francisco for the measurements, computing and statistics in the work.
BIBLIOGRAPHIC REFERENCES
1. Parker DJ, Cantrell JW, Karp RB, Stroud RM, Digerness SB. Changes in serum complement and immunoglobulins following cardiopulmonary bypass. Surgery 1972; 71:824-7.
[ Medline ]
2. Gomes WJ, Carvalho AC, Palma JH, Gonçalves Jr. I, Buffolo E. Vasoplegic syndrome: a new dilemma. J Thorac Cardiovasc Surg 1994; 107:942-3.
[ Medline ]
3. Andrade JCS, Batista Filho ML, Évora PRB, Tavares JR, Buffolo E, Ribeiro EE et al. Utilização do azul de metileno no tratamento da síndrome vasoplégica após cirurgia cardíaca. Rev Bras Cir Cardiovasc 1996; 11:107-14.
[ Lilacs ]
4. Kofidis T, Strüber M, Wilhelmi M, Anssar M, Simon A, Harringer W et. al. Reserval of severe vasoplegia with single-dose methylene blue after heart transplantation. J Thorac Cardiovasc Surg 2001; 122:823-4.
5. Koelzow H, Gedney JA, Baumann J, Snook NJ, Bellamy MC. The effect of methylene blue on the hemodynamic changes during ischemia reperfusion injury in orthotopic liver transplantation. Anesth Analg 2002; 94:824-9.
[ Medline ]
6. Grayling M, Deakin CD. Methylene blue during cardiopulmonary bypass to treat refractory hypotension in septic endocarditis. J Thorac Cardiovasc Surg 2003; 125:426-7.
[ Medline ]
7. Salaris SC, Babbs CF, Voorhees 3rd WD. Methylene blue as an inhibitor of superoxide generation by xanthine oxidase: a potential new drug for the attenuation of ischemia / reperfusion injury. Biochem Pharmacol 1991; 42:499-506.
[ Medline ]
8. Finkel MS, Oddis CV, Jacob TD, Watkins SC, Hattler BG, Simmons RL. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science 1992; 257:387-9.
[ Medline ]
9. Mayer B, Brunner F, Schmidt K. Inhibition of nitric oxide synthesis by methylene blue. Biochem Pharmacol 1993; 45:367-74.
[ Medline ]
10. Brasil LA, Gomes WJ, Salomão R, Buffolo E. Inflammatory response after myocardial revascularization with or without cardiopulmonary bypass. Ann Thorac Surg 1998; 66:56-9.
[ Medline ]
11. Chenoweth DE, Cooper SW, Hugli TE, Stewart RW, Blackstone EH, Kirklin JW. Complement activation during cardiopulmonary bypass: evidence for generation of C3a and C5 a anaphylatoxins. N Engl J Med 1981; 304:497-503.
[ Medline ]
12. Cremer J, Martin M, Redl H, Bahrami S, Abraham C, Graeter T et al. Systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg 1996; 61:1714-20.
[ Medline ]
13. Diegeler A, Doll N, Rauch T, Haberer D, Walther T, Falk V et al. Humoral immune response during coronary artery bypass grafting: a comparison of limited approach, "off-pump" technique, and conventional cardiopulmonary bypass. Circulation 2000; 102 [19 suppl 3]: III 95-100.
14. Edmunds Jr. LH. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg 1998; 66 (5 suppl) S12-6.
15. Holmes JH, Connolly NC, Paull DL, Hill ME, Guyton SW, Ziegler SF et al. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res 2002; 51:579-86.
[ Medline ]
16. Taylor KM. SIRS: the systemic inflammatory response syndrome after cardiac operations. Ann Thorac Surg 1996; 61:1607-8.
[ Medline ]
17. Westaby S. Complement and the damaging effects of cardiopulmonary bypass. Thorax 1983; 38:321-5.
[ Medline ]
18. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992; 101:1644-55.
[ Medline ]
19. Évora PR. Should methylene blue be the drug of choice to treat vasoplegias caused by cardiopulmonary bypass and anaphylactic shock? J Thorac Cardiovasc Surg 2002; 119:632-4.
20. Keaney Jr. JF, Puyana JC, Francis S, Loscalzo JF, Stamler JS, Loscalzo J. Methylene blue reverses endotoxin-induced hypotension. Circ Res 1994; 74:1121-5.
[ Medline ]
21. Preiser JC, Lejeune P, Roman A, Carlier E, De Backer D, Leeman M et al. Methylene blue administration in septic shock: a clinical trial. Crit Care Med 1995; 23:259-64.
[ Medline ]
22. Schneider F, Lutun P, Hasselmann M, Stoclet JC, Tempé JD. Methylene blue increases systemic vascular resistance in human septic shock: preliminary observations. Intensive Care Med 1992; 18:309-11.
[ Medline ]
23. Weingartner R, Oliveira E, Oliveira ES, Sant´Anna UL, Oliveira RP, Azambuja LA et al. Blockade of the action of nitric oxide in human septic shock increases systemic vascular resistance and has detrimental effects on pulmonary function after a shost infusion of methylene blue. Braz J Med Biol Res 1999; 32:1505-13.
[ Medline ] [ Lilacs ] [ SciELO ]
24. Brett SJ, Quinlan GJ, Mitchell J, Pepper JR, Evans TW. Production of nitric oxide during surgery involving cardiopulmonary bypass. Crit Care Med 1998; 26:272-8.
[ Medline ]
25. Aldea GS, Soltow LO, Chandler WL, Triggs CM, Vocelka CR, Crockett GI et al. Limitation of thrombin generation, platelet activation, and inflammation by elimination of cardiotomy suction in patients undergoing coronary artery bypass grafting treated with heparin-bonded circuits. J Thorac Cardiovasc Surg 2002; 123:742-55.
[ Medline ]





 All scientific articles published at rbccv.org.br are licensed under a Creative Commons license
All scientific articles published at rbccv.org.br are licensed under a Creative Commons license